ChemInform Abstract: Poly-Organo-Lithium Compounds. Part 15. Reductive Cleavage of a Cyclobutane σ-Bond and Rearrangement Involving 1,7-Proton Shift of the Initially Formed 1,4-Dilithio Compound in the Reaction of Diphenylmethylenecyclobutane with Elemental Lithium.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
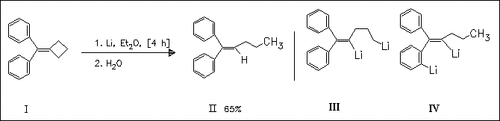
The title reaction of the compound (I) leads to a reductive cyclobutane σ-bond cleavage affording the product (II). As it is evident from a similar reaction using D2O instead of H2O in the hydrolysis step, the initially formed dilithio compound (III) slowly undergoes rearrangement with 1,7-proton shift yielding another, presumably doubly-bridged 1,4-dilithio compound (IV), which is rather stable in Et2O.