ChemInform Abstract: Approaches to Selective Isoprenologation via Reactions of (η3- Allyl)Fe(CO)+ 4 with Allyl Nucleophiles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
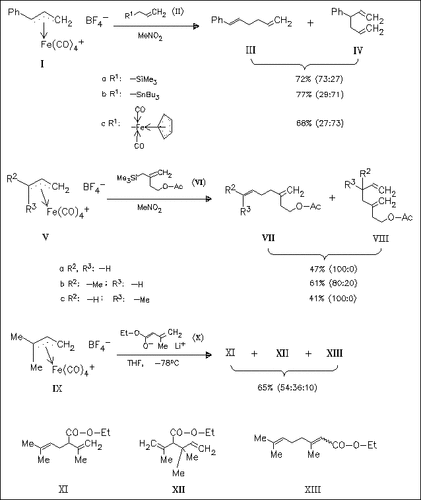
The regioselectivity in the allyl-allyl coupling between the iron complex (I) and the metal allyls (II) is found to depend on the metal used. The reaction of the iron complexes (V) with the silyl derivatives (VI) generally provides good regioselectivity in favor of the linear 1,5-dienes (VII) resulting from attack at the C-3 position of (V) needed for applications in isoprenologation. Coupling of the iron complex (IX) with the lithium dienolate (X), however, gives mainly the products (XI) and (XII) resulting from attack at the α -position of the dienolate, but acceptable levels of the terminal . gamma.-attack product (XIII) have yet to be found.