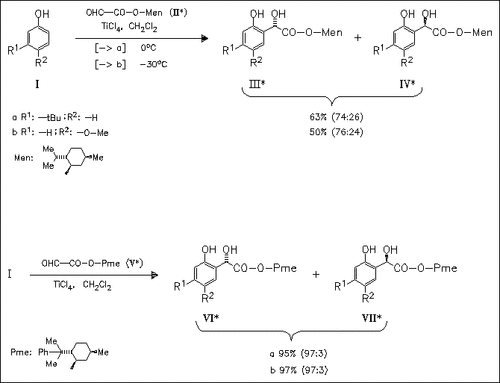
ChemInform Abstract: Highly Regio- and Diastereoselective Friedel-Crafts Alkylation of Phenols. Synthesis of 2-Hydroxymandelic Esters.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The use of mandelic acid as a drug and the interest in asym. electrophilic aromatic substitution results in the synthesis of the title esters (III), (IV), (VI) and (VII). The diastereomeric ratio depends on the bulkiness of the chiral esters (II) and (V) and on the Lewis acid used. Best results are achieved with TiCl4.