ChemInform Abstract: Nucleosides. Part 153. Synthesis of 1-Methyl-5-(3-azido-2,3-dideoxy-. beta.-D-erythro-pentofuranosyl)uracil and 1-Methyl-5-(3-azido-2,3- dideoxy-2-fluoro-β-D-arabinofuranosyl)uracil. The C-Nucleoside Isostere of 3′-Azido-3′-deoxythymidine and Its 2′-“Up”-Fluoro Analogue.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
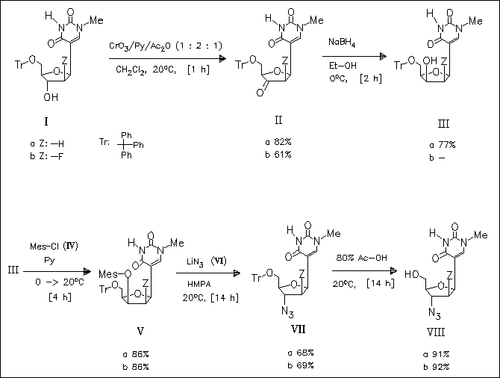
The compounds (VIII) mentioned in the title are synthesized as outlined in the reaction scheme. Other attempts to get these compounds using different synthetic routes are also described. (VIIIa) is a C- nucleoside isostere of the potent anti-AIDS nucleoside AZT. However, neither (VIIIa) nor (VIIIb) show any significant inhibitory activity against HIV in H9 cells.