ChemInform Abstract: Synthesis and Biological Activity of Novel 5-(2-Acylethynyl)uracils.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
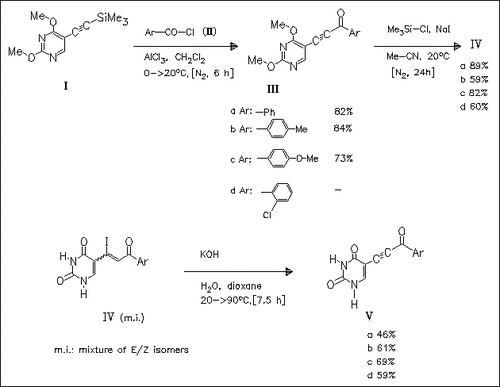
Acylation of 2,4-dimethoxy-5-(2-trimethylsilylethynyl)pyrimidine (I) with the acid chloride (II) produces the acyl derivatives (III) which are converted to the corresponding 5-(2-acyl-1-iodovinyl)uracils (IV) by reaction with chlorotrimethylsilane and sodium iodide. Treatment of (IV) with potassium hydroxide yields the title compounds (V) which are active against Ehrlich ascites carcinoma cells in vivo. They also display in vitro activity against CCRF-CEM and L1210/0 tumor cell lines.