ChemInform Abstract: gem-Dialkyl Effect in the Intramolecular Diels-Alder Reaction of 2- Furfuryl Methyl Fumarates: The Reactive Rotamer Effect, Enthalpic Basis for Acceleration, and Evidence for a Polar Transition State.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
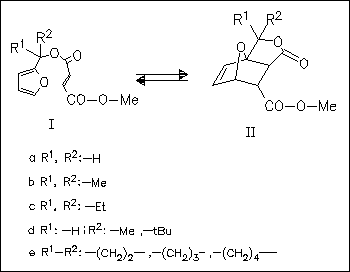
The gem-dialkyl effect (acceleration of a cyclization due to the substitution of alkyl groups for hydrogen atoms on the carbons in the chain that links the two reactive centers) is investigated by determination of the activation parameters for the intramolecular Diels-Alder reaction (I) → (II). The results indicate that the gem-dialkyl effect is caused not by angle compression but by some other effect of dialkyl substitution at a central methylene on the linking chain, namely the reactive rotamer effect. A strong acceleration of the cycloaddition in polar solvents is explained by polar transition states.