Thermally insulating and fire-retardant bio-mimic structural composites with a negative Poisson's ratio for battery protection
Abstract
Battery safety has attracted considerable attention worldwide due to the rapid development of wearable electronics and the steady increase in the production and use of electric vehicles. As battery failures are often associated with mechanical-thermal coupled behaviors, protective shielding materials with excellent mechanical robustness and flame-retardant properties are highly desired to mitigate thermal runaway. However, most of the thermal insulating materials are not strong enough to protect batteries from mechanical abuse, which is one of the most critical scenarios with catastrophic consequences. Here, inspired by wood, we have developed an effective approach to engineer a hierarchical nanocomposite via self-assembly of calcium silicate hydrate and polyvinyl alcohol polymer chains (referred as CSH wood). The versatile protective material CSH wood demonstrates an unprecedented combination of light weight (0.018 g cm−3), high stiffness (204 MPa in the axial direction), negative Poisson's ratio (−0.15), remarkable toughness (6.67 × 105 J m−3), superior thermal insulation (0.0204 W m−1 K−1 in the radial direction), and excellent fire retardancy (UL94-V0). When applied as a protective cover or a protective layer within battery packages, the tough CSH wood can resist high-impact load and block heat diffusion to block or delay the spread of fire, therefore significantly reducing the risk of property damage or bodily injuries caused by battery explosions. This work provides new pathways for fabricating advanced thermal insulating materials with large scalability and demonstrates great potential for the protection of electronic devices.
1 INTRODUCTION
Batteries in aerospace, portable electronics, and electrical vehicles have raised significant safety concerns due to their potential risk of fire/explosion problems, and thousands of accidents related to battery failures are reported worldwide each year.1-3 For example, burning and smoking batteries led to the grounding of the world's most advanced Boeing 787 airliners, Tesla Model S car batteries caused devastating house fires, and the explosion of cell phone batteries resulted in the recall of Samsung Galaxy Note 7.4 These incidents have attracted mass public attention and reminded us continuously that safety is a prerequisite for any electronic equipment. Mechanical and thermal problems are major reasons that cause catastrophic events.5-7 Therefore, effective thermal insulation materials with mechanical stability to protect battery packages are highly desired to ensure electric safety.8, 9 However, for advanced thermal insulators, achievement of a perfect combination of low density (light weight for portable devices), high mechanical strength (to defend against mechanical shock), and low thermal conductivity (to avoid thermal runaway) is a huge challenge.10 Current thermal insulation materials have certain intrinsic defects; for example, inorganic aerogels (silicate or SiC) are too weak to tolerate mechanical collision,11 polymer insulators are flammable and directly lead to overheating problems,12, 13 and carbon composites undergo structural shrinkage and involve the use of complicated fabrication processes.13
Wood is an emerging candidate that has both superior mechanical properties and excellent thermal insulation owing to its hierarchical structure and well-oriented matrix.14 The hollow channels endow the wood with high porosity and low density, which contribute to the anisotropic structure, and enable redirection of thermal energy and prevention of heat localization through multiple fibril layers (perpendicular to the walls).15 In the meantime, outstanding mechanical strength could be obtained along the channel direction.16
Inspired by the unique microstructures of natural wood, many polymer-based synthetic materials with well-defined architectures have been fabricated with high mechanical toughness and exceptional thermal insulation performance. However, ignition is still a major weakness in such insulators, and thus, the addition of flame retardants is required. Unfortunately, many of the commonly used coating and flame retardants are phosphorous or halogenated, with raising major concerns in relation to human health and the environment.17 To improve fire retardancy, inorganic nanocomposites (nanoclays or graphene-based nanofillers) are used to provide fire barriers and reinforce the skeletons of polymeric matrices.18, 19 However, satisfactory protection against both fire and mechanical abuse cannot be achieved due to the weak interfacial interactions between the fillers and the polymer matrix.
Calcium silicate hydrate (C–S–H), the green nanoparticles that have a strong cohesive force to drive cement (the most widely used substance in the world20, 21) into hard solid binding blocks,22, 23 is very attractive in terms of improving both mechanical and flame-retardant properties of wood-like structured materials because it can cross-link the polymer chains to form a 3D nanostructure and simultaneously generate an effective protective layer on the burning surface.24, 25 Moreover, the in situ assembly of ultrathin nanosheets of C–S–H could result in a well-distributed framework with a large specific surface area, devisable pore volume, and interconnected nanochannels, which is therefore an ideal material for thermal insulation.26-29
Herein, we have developed a novel anisotropic lamellar CSH–polyvinyl alcohol (PVA) composite (CSH wood) with the properties of low density, excellent mechanical performance, and low thermal conductivity using a tailorable freeze-drying process. The choice of flexible PVA chains and the addition of C–S–H reduce the volume shrinkage and promote scale-up fabrications. Environmentally friendly C–S–H nanoparticles also contribute to the high mechanical stiffness and excellent toughness of the composites. Therefore, CSH wood had a perfect combination of superior thermal insulating properties (0.0204 W m−1 K−1), ultralow density (18 mg cm−3), and high mechanical strength (204 MPa). When applied as a battery protection shield, it can insulate a burning battery at an extremely high temperature of 867.5°C and maintain the outside temperature at 36.2°C. The structural design and engineering technique of CSH wood provide new pathways for fabricating advanced thermal insulating materials and demonstrate great potential for addressing the fire and exploration problems hidden in electronic devices.
2 EXPERIMENTAL SECTION
2.1 Materials
Polyvinyl Alcohol (PVA~224, Mw~205,000), calcium nitrate (Ca(NO3)2·4H2O), and sodium silicate (Na2SiO3·9H2O) were provided by Shanghai Macklin Biochemical Co., Ltd. A lithium-ion battery (NCR 18650PF, 2900 mAh, 3.7 V) (18 mm diameter × 65 mm height) and a lithium-ion battery (NCM 523, 100 Ah, 3.65 V) (148 mm × 52 mm × 95 mm) were purchased from CATL Co., Ltd.
2.2 Fabrication
Three precursors were prepared in the spinning solution: PVA, Ca(NO3)2·4H2O, and Na2SiO3·9H2O. First, PVA at different content (3 wt%, 7.5 wt%, 10 wt%) was dissolved in deionized water by magnetic stirring at 90°C for 3 h. Different concentrations of Ca(NO3)2 solution (0.2, 0.45, 0.9 M) were added to the PVA solution for cross-linking. Then, the Na2SiO3 solution was subsequently added dropwise to the mixed solution. The molar ratio of Na2SiO3 and Ca(NO3)2 is 1:1, and the concentration of Na2SiO3 is maintained at 0.2, 0.45, or 0.9 M for different CSH wood samples. The PVA is applied as a tackifier to promote C–S–H gelation and a template to regulate the formation of C–S–H nanoparticles during the self-assembly process. Therefore, its content is needed to be more than 3% to maintain the structural integrity of the polymer skeleton. The C–S–H nanoparticles serve as the calcium source to cross-link the PVA polymer chains via coordination bonds, which contributes to the mechanical robustness and fire resistance of CSH wood. However, the concentration of Ca(NO3)2 and Na2SiO3 for in situ synthesis of C–S–H should not exceed 2 mol L−1 to avoid agglomeration in the precursors. The transparent solution gradually became milky white with the addition of sodium silicate. The chemical equation is Ca2+ + SiO32− + H2O → (CaO)xSiO2(H2O)y. The precursors were pre-frozen in a liquid nitrogen bath for 20 min to control the directional growth of ice crystals, and then frozen dry at −101°C for 12 h to obtain CSH wood.
2.3 Characterizations
The compressive tests were performed using a universal UTM5105 testing machine with a 5000 kN load cell. The loading rate was set at 3 mm min−1. The microscopic morphologies of the aerogel were examined using a field emission scanning electron microscope (ZEISS Gemini 300). The elemental composition was examined by energy-dispersive spectroscopy. The distribution of layered microstructures was determined by analyzing the scanning electron microscope (SEM) images using Image-Pro Plus software (Media Cybernetics). The thermal conductivity of CSH wood (20 mm × 20 mm × 20 mm) was determined by thermal conductivity analyses (TPS 2500S; Hot Disk Instruments) using the transient plane source method according to the testing standard of ISO 22007-2:2015. All the Li-ion batteries are new and all the experiments were conducted at a room temperature of 20°C. The state of charge (SOC) of the NCR 18650PF Li-ion battery is 2900 mAh and 3.7 V, and that of the NCM 523 Li-ion battery is 100 Ah and 3.65 V. The K-type thermocouples attached on the side surface of batteries were applied to ensure continuous heat and their constant output power was 50 V, 250 W. The samples were crushed into small fragments for observation using an X-ray diffractometer (Burker D8 Advance X) equipped with Cu Kα radiation (λ = 0.15406 nm) with 2θ ranging from 5° to 80° at a 10° min−1 scanning rate. The density of CSH wood was calculated using the measured mass and geometry of each sample. Infrared (IR) spectra were recorded using a Fourier transform infrared spectrometer (FTIR) spectrometer (Bruker Vertex 70) at room temperature. The viscosity rate was determined using a rheometer (Physica MCR 302; Anton Paar) with a 0.5 N prestress and 1% shear strain at an angular frequency of 0.5–200 rad s−1. The parameters related to porosity and tortuosity were measured using a Mercury Porosimeter (Auto pore IV 9520). Solid-state 29Si spectra were recorded on an Agilent 600 DD2 spectrometer (Agilent, magnetic field strength 14.1 T) at a resonance frequency of 199.13 MHz for 29Si. The IR thermal images of the samples were captured by an IR thermal camera (FLTR T540). The battery explosion experiments are set up in a standard laboratory environment, including temperature sensor layout, and the typical trigger equipment according to GB/T 2900.41-2008 and GB/T 31 485-2015.
2.4 Molecular dynamics (MD) simulations
A model cube was set up with dimensions of a = 21.38 Å, b = 20.60 Å, and c = 34.26 Å. A large-scale atomic/molecular massively parallel simulator (LAMMPS) was utilized to carry out the simulation. In this simulation, two force fields, the clay force field (CLAYFF) and the consistent valence force field (CVFF), were packed into the interactions during the simulation process. Specifically, the former is suitable for the analysis of C–S–H, and the latter is used to describe PVA. The interaction parameters between C–S–H and PVA atoms were calculated based on the mean rule, including the arithmetic mean rule for distance parameters and the geometric mean rule for energy parameters. Additionally, a hood canonical ensemble (NVT) at 298 K was chosen in the whole simulation. The production period lasted 500 ps after a 100 ps equilibration time, and the data were collected every 1 ps during this period to examine the interactions.
2.5 Simulation of the physical process
All the finite element method (FEM) simulation results in this paper were obtained using ABAQUS software. We created a porous structure with 30 cm length × 30 cm height × 2 cm weight to examine the negative Poisson's ratio (NPR) behavior under the deformation process in the solid mechanical field.
2.6 Battery explosion experiment
The battery explosion test was carried out in a strict laboratory environment, standard temperature sensor layout, and typical trigger equipment (Chery Automobile Co., Ltd.) according to GB/T 2900.41-2008 and GB/T 31485-2015.
3 RESULTS AND DISCUSSION
3.1 Microstructure of CSH wood
The design and development strategy of bioinspired CSH wood are based on a facile and efficient ice-template-induced directional crystallization method (Figure 1). Due to the temperature gradient, the ice crystals grow from the bottom to the top. The emulsion particles in the mixed dispersion are then ejected by the growing crystals. After coalescence and sublimation, a well-designed 3D laminar structure is formed. PVA is not only used as a template to form highly oriented C–S–H nanoparticles but also a tackifier to promote C–S–H gelation during the self-assembly process. A detailed analysis of the viscosity difference based on the rheometer results is shown in Figures S1 and S2. The C–S–H nanoparticles serve as the source of calcium to cross-link the PVA chains via coordination bonds, which contributes to the mechanical robustness of CSH wood.
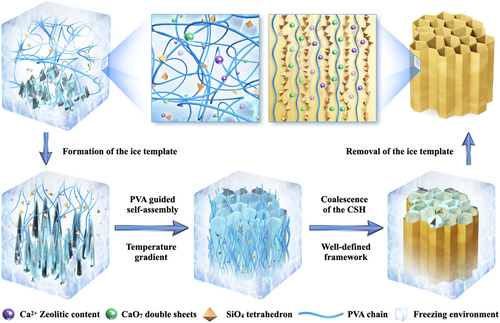
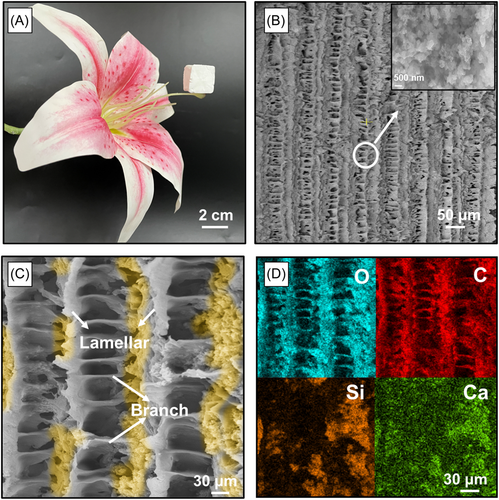
After removing the ice template using the freeze-drying method, the resulting CSH wood presented a hierarchical porous structure with C–S–H wrapped on the surface of the laminar walls. It had an ultralow density of 18.3 mg cm−3, with a porosity of up to 89.85% (Table S1), and could freely stand on the pistil of a flower (Figures 2A and S3). The whole fabrication process takes about 2 days in the laboratory, which could be significantly shortened and easily scaled up with the use of industrial equipment. The precise control and delicate design of wood-mimetic materials in our approach are reflected in the microstructure, specifically the pore size and lamellar thickness. The SEM image in Figure 2B shows the perfect hierarchical architectures with interconnected bridges in the cross-section of CSH wood, which is consistent with the unique micro-oriented and macro-anisotropic nature of natural wood. Figure 2C shows that the organized lamellar-cell geometry architecture consists of three parts: lamellar walls, side branches, and pores. The picture in the inset shows the nanopore microstructure of CSH wood. The lamellar structure and side branches generated during freezing could be tuned by adjusting the concentrations of PVA and C–S–H. For better comparison, we conducted a series of experiments to investigate the pore size evolution and lamellar thickness distributions based on the cross-section morphology. Figure S4 summarizes the variation of microscopic morphology at different precursor concentrations. At low concentrations (Figure S4a,d,g), the dendrites disappeared after freeze-drying, while the concentration increased, and the particles were more likely to be trapped as ice crystals grew, resulting in more interconnected structures. A higher filler content led to an increase in the nucleation density of ice crystals in the cooling stage, leading to a narrower pore size with a more obvious fishbone structure in the final CSH wood (Figure S4c,f,i). The corresponding energy-dispersive spectrum (EDS) mapping results (Figures 2D and S5) show that C–S–H particles are highly entangled upon the lamellar surface, and the Ca and Si elements are closely packed with the C and the O elements, revealing that the oriented C–S–H nanoplates are distributed in an orderly manner on the surface of the PVA skeleton at the mesoscopic scale.
As can be seen from the X-ray Diffraction (XRD) results shown in Figure S6, the characteristic peaks at 19.17° and 39.74° correspond to the PVA polymer. The peaks at 32.22° and 48.19° confirm the presence of Ca(OH)2, and the peak at 29.1° is determined to be C–S–H. The FTIR spectra of CSH wood are presented in Figure S7, where strong Si–O–Si asymmetric stretching vibrations at 1095 and 1335 cm−1 as well as a relatively weak peak of the Si–O–C bond at 1145 cm−1 are detected, demonstrating the chemical interactions between the PVA and C–S–H.30 This chemical bonding between the PVA chains and the neighboring silicate sheets could promote the nucleation of C–S–H. The results from 29Si nuclear magnetic resonance (NMR) further confirm the interaction between silicates from C–S–H and PVA. The addition of PVA changes the Si composition of C–S–H, decreasing the percentage of the Q1 structure (located at −76 ppm) of silicate tetrahedra and increasing the proportion of the Q2 structure (located at −86 ppm) (Figure S8 and Table S2). The survey X-ray photoelectron spectroscopy (XPS) in different types of CSH wood is shown in Figure S9. Depth profiling of O1s, C1s, Ca2p, and Si2p demonstrates the existence of hydrogen bonding and calcium coordination interactions between PVA and C–S–H, as calcium exists as Ca2+ (350.72 eV) and Ca–O (347.19 eV), and silicon exists as Si–O (103.32 eV) and organic Si (101.89 eV).
3.2 Mechanical performance and failure mechanism
To protect batteries from mechanical crushing, high stiffness and strong compression and impact resistance are the most desirable advantages. Compared with classic ceramic aerogels with brittleness, the acquired CSH wood shows structural robustness that can tolerate large compression without cracking. It can bear 20,000 times its weight without any deformation (Figure 3A). The obtained stress–strain measurements presented in Figure 3B show the typical anisotropic deformation behavior of CSH wood. The axial compressive curve showed the characteristics of open honeycomb-like foams, yielding three stages: an elastic regime, a stress plateau, and high densification. The radial curve showed a layer-by-layer failure mechanism: periodic fluctuation. The axial compressive strength was much higher than the radial compressive strength, and a large elastic modulus of 204 MPa along the axial direction was obtained. Compared with other foams and aerogels reported in the literature (Figure 3C), CSH wood demonstrated the best elastic modulus in the same density range. As shown in Figure S10, the ultimate tensile strength of CSH wood is 8.74 MPa, with the composition of 7.5% PVA, 0.45 M Ca(NO3)2, and 0.45 M of Na2SiO3. The 9 mm CSH wood was subjected to a puncture experiment with a 25-gauge needle at a speed of 40 mm min−1, while the maximum load stress reached 1320.9 N.
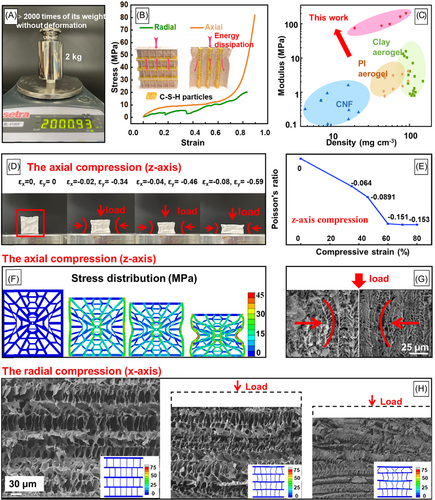
Due to the anomalous microstructure, CSH wood could retain an NPR (from 0 to −0.15). As shown in Figure 3D,E and Movie S1, the strain evolution of CSH wood ranged from 0 to −0.08 on the z-axis and from 0 to −0.59 on the y-axis. To understand the failure mechanism of CSH wood, FEM simulation was applied to restore the mechanical compression process (Figure 3F), and the simulation results indicated special hyperbolic-patterned shrinkage, which revealed the obvious NPR behavior. As shown in the SEM images of Figure 3G, similar hyperbolic-patterned deformation in the z-axis was observed, confirming that the structure with NPR behavior is superior in absorbing energy during compression. During x-axis radial loading, the stress–strain curve has periodic fluctuations; the series of cross-sectional deformation images in Figure 3H shows that the side branches also served as the main support and exhibited characteristics of layer-by-layer failure.
The drop-tower impact test demonstrated that CSH wood maintained its structural integrity without any deformation when a steel ball (200 g, 200 times heavier than the weight of the sample) was released from a height of 200 mm (impact energy = 400 mJ) (Figure 4A and Movie S2). Due to the well-designed porous cellular structure and NPR, CSH wood achieved an exceptionally high toughness. For comparison with other high toughness aerogels, we integrated a similar strain and obtained 667.56 mJ cm−3 toughness in our CSH wood by integrating the stress–strain curve. It manifests much higher toughness than other rigid aerogels, whose toughness are smaller than 100 mJ cm−3 (Figure 4B).
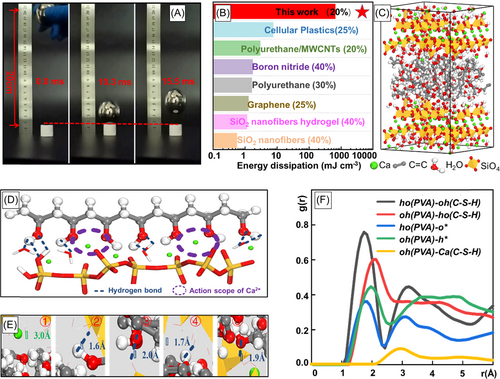
We believe that both lamellar-cell architecture and well-bonded chemical phases maximize the toughness, and the strengthening mechanism of the C–S–H particles and PVA chains was investigated by MD simulations. As shown in Figure 4C, the modeling structures of CSH wood were created and stretched along the z-axis, and the local magnified schematic interfacial structure of PVA/C–S–H is presented in Figure 4D. The C-S-H particles are connected on the surface PVA chains due to the formation of hydrogen bonding and the coordination of calcium ions (Figure 4E). The hydrogen atom from the PVA chains attracts –OH from the C–S–H matrix, corresponding to the first type of hydrogen bond (the first peak in Figure 4F). Similarly, –OH groups from PVA are combined with the hydrogen atoms from C–S–H nanoplates, referring to red line in Figure 4F. Furthermore, calcium ions in the interlayer not only coordinate with the oxygen atoms from silicates but also attract oxygen from PVA groups (Figure 4E, dashed green circles), shown by the yellow curve in Figure 4F, confirming the affinities between the organic and inorganic phases.
For the durability experiment, the CSH wood sample was placed in air at 25°C for 2 months, and the weight remained unchanged. Moreover, its weight retention rate increased to 101.11% (Figure S11) when it was placed in a humidity chamber (T = 30°C, RH = 50%) for 30 days, indicating its superior environmental sustainability and durability. This high performance could be ascribed to the cross-linking of C–S–H and the polymer chains, which form a 3D nanostructure and generate an effective protective layer on the PVA surface simultaneously. In addition, the robust structure (Ca–O sheets sandwiched between silicate chains) and the hardening ability (mechanical strength getting harder with increasing time) of the three-dimensional C–S–H nanocrystals significantly improve the composite's chemical stability against humid environments.20, 30, 40
3.3 Anisotropic thermal insulation property
In addition to excellent mechanical properties, thermal insulation is another important parameter for protective covers to improve the safety of electronic equipment. As shown in Figures 5A and S5, porous CSH wood has two levels of porosity, including micro-scale pores ranging from 10 to 30 μm formed by the PVA skeleton and nano-scale pores ranging from 10 to 50 nm formed by the self-assembly of C–S–H particles, which could induce the Knudsen effect and retain the heat flow within multiple pores.41 For the samples with the same PVA concentration, CSH wood showed decreasing thermal conductivity with increasing C–S–H content due to the formation of a progressively enhanced multilayer structure (Figure S4a,b,c).
CSH wood acquires a low thermal conductivity of 0.0301 W m−1 K−1 at a C–S–H concentration of 0.45 mol L−1. Thermal anisotropy obtained along different directions of CSH wood is also shown in Figure 5A. The thermal conductivity in the axial direction is obviously higher than that in the radial direction. The thermal conductivity in the radial direction considerably decreases from 0.0305 to 0.0204 W m−1 K−1 (significantly lower than that of air) when the temperature increases from 20°C to 80°C (Figure 5B). The dynamic temperature distributions were monitored using an IR camera and are presented in Figures 5C and S12. When the bottom was heated at 200°C, the top surface temperature increased from 28°C to 30°C after 30 min and remained the same after 1 h. The side surface temperature in Figure S12 is higher due to the absence of multiple frameworks.
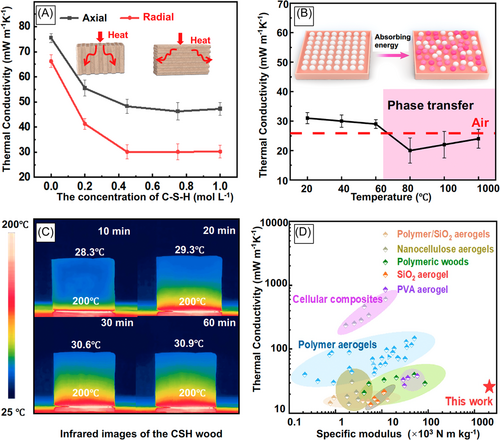
This significant decrease in thermal conductivity at approximately 80°C can be explained by the glass transition phenomenon. In the glass transition temperature range, the atomic chains tend to disentangle and gain more space for thermal movement. This transition contributes to more intensive thermal energy absorption.52 The step-like curves of the differential scanning calorimetry measurement shown in Figure S13 show the endothermic glass transition during the heating process. We attributed the ultralow thermal conductivity of CSH wood to the following reasons: (1) the in situ-generated C–S–H nanoparticles with a pore size of 1–50 nm serve as thermal barriers to slow down the thermal transmission (Figure S5); (2) phonons propagate less effectively through hydrogen bonds; (3) CSH wood undergoes glass transition at around 80°C, and the atomic chains tend to disentangle and obtain more space for thermal movement; and (4) the laminated structure contributes toward redirecting heat flow and preventing local overheating in the direction perpendicular to the channel, decreasing the overall thermal conductivity (Figure S14).16, 45, 51, 53 The comparisons of CSH wood with other thermal insulations are shown in Figure 5D. The thermal insulation and specific modulus of our CSH wood are much higher than those of most state-of-the-art aerogels. In particular, the modulus of CSH wood is one order of magnitude better than that of polymeric wood. Although ceramic aerogels are attractive for thermal insulation, they have the major limitations of severe strength degradation and structural collapse. Our CSH wood is lightweight, tough, and thermally resistant, and could provide very safe protection for batteries in abusive conditions.
3.4 Fire retardancy and battery protection
Most wood-derived materials show excellent mechanical performance and thermal insulation, but typically have the disadvantage of flammability. Therefore, our CSH wood has been engineered to combine remarkable structural robustness with outstanding flame-retardant properties for battery protection applications. The limiting oxygen index (LOI) and horizontal burning test (HB-UL94) measurements are applied to evaluate the fire retardancy properties of CSH wood. It has a significantly higher LOI value (46%) than commercial polymeric foams (21%).45, 54 A horizontal combustion experiment was conducted to examine the combustion range and flame propagation velocity of CSH wood. No obvious damage can be observed after 40 s of ignition (Figure 6A and Movie S3). This can be attributed to the fact that the inorganic CSH nanocrystals not only formed an interconnected network in the polymeric matrix but also wrapped on the burning surface of the laminar PVA walls, which can significantly improve the fire retardancy of CSH wood.
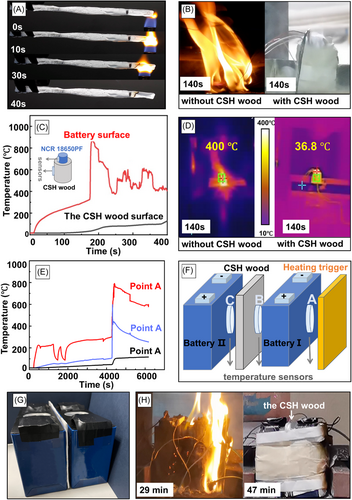
Battery explosion experiments were conducted to test the protective performance of CSH wood, including the all-inclusive shield over a single lithium-ion battery (NCR 18650PF) and the laminates separated between two LIB packages (NCM 523) according to the GB/T 2900.41-2008 and GB/T 31485-2015 standards.55, 56 The complete processes were recorded, and they can be found in Movies S4 and S5 and are summarized in Table S3. As shown in Figure 6B, the battery without the shield has a large fire flame, while the battery covered with CSH wood protection only emits white smoke without any fire flame. Figure 6C,D shows the large temperature differences of the batteries with and without the CSH wood cover during the same heating procedure. When the temperature of the battery rapidly increases to almost 1000°C (Point A), the temperature of CSH wood remains at 36.8°C. The bare battery started to burn after 2 s of heating and continued to burn for over 200 s, but the battery with CSH wood started to smoke after 276 s and no fire was observed. Our CSH wood can prevent combustion and maintain the structural integrity of the LIBs, which is in urgent demand for portable and wearable electronics.
We adopted two parallel NCM 523 battery packages as experimental samples (Figure 6E,F). Heating was triggered on one side surface of an individual battery package with 50 V and 350 W power. Temperature sensors were placed in three positions to monitor the protection performance of CSH wood (Figure 6F). The CSH wood protective layer with excellent thermal insulation (0.0204 W m−1 K−1) can redirect the heat flow and prevent local overheating on the other battery, therefore preventing thermal runaway propagation to the adjacent cell. When the temperature increased to 789°C at point A and 613.5°C at point B, the battery separated by CSH wood remained at 100°C at Point C (Figure 6E). The CSH wood block thermal runaway from the cell to the surroundings, protecting the battery pack from collateral damage. When one single cell overheats and burns, the CSH wood laminates can prevent heat transfer to the other cells, significantly delaying the spread of the fire and providing enough time to escape and rescue (Figure 6G,H).
Compared with the widely used battery packaging materials in electric vehicle industries, sponge foam and plastic foams have the disadvantage of large densities (higher than 200 mg cm−3);57, 58 our superlight CSH wood can effectively block heat diffusion from a single out-of-control cell, reducing the risk of disasters with a density of only 18.3 mg cm−3, and thus significantly boost the energy density of the whole battery package. As a result, the superior lightweight and high energy-absorbing features make CSH wood an ideal candidate that can be used to improve the safety of existing battery systems.
4 CONCLUSIONS
In summary, we have developed a wood-like hierarchical porous architecture with prominent anisotropy through tailorable freeze-drying of PVA and nano C–S–H. In the axial direction, the ultralight CSH wood shows a strong stiffness of 204 MPa and an NPR of −0.15. In the radial direction, remarkable thermal insulation performance is achieved at 0.0204 W m−1 K−1. Moreover, robust CSH wood demonstrates the best toughness of 6.67 × 105 J m−3 among many other materials reported in the literature. When applied as a battery protection cover or layer, CSH wood showed superior fire retardancy properties, preventing combustion and maintaining the structural integrity of the batteries after explosions. It can tolerate the inside temperature of 867.5°C and maintain its outside surface temperature at 36.5°C, significantly delaying the spread of the fire. The unique comprehensive features of superior light weight, high stiffness, excellent toughness, outstanding thermal insulation, and fire-resistance properties make CSH wood an ideal protection material for many electronic devices.
ACKNOWLEDGMENTS
The authors would like to acknowledge the financial support from the National Key Research and Development Program of China (No. 2021YFF0500802) and the National Natural Science Foundation of China (No. 51890904, No. 52022022, and No. 52278247) and the Scientific Research and Innovation Plan of Jiangsu Province (KYCX21_0090). Thanks are due to Zhangli Hu from Southeast University for her assistance with the mechanical analysis, and Xiaoyu Chen and Xiangbing Zeng from Chery Automobile Co., Ltd. for their assistance on the battery explosion experiment. The authors would like to thank Jiangsu Sobute New Materials Co., Ltd. for the test site and Shiyanjia Lab (www.shiyanjia.com) for the NMR analysis.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




