Cannabigerol Extraction from a Cannabis Cultivar Using a Supercritical Carbon Dioxide Process
Abstract
To attempt a selective extraction from a cannabigerol (CBG)-rich Cannabis cultivar, supercritical fluid extraction was tried, operating at 18 MPa and 40 °C. In the first set of experiments, a fractional extraction scheme was used to favor the separation between the cannabinoid extract and paraffinic waxes: global and CBG yields were equal to 4.8 % and 3.8 % w/w, respectively, leading to an overall CBG concentration in the extract up to 79 %. However, GC–MS traces showed that CBG also tended to precipitate in the refrigerated separator together with paraffinic waxes, due to CBG steric configuration that is very similar to the one of paraffins. Therefore, non-fractional extraction experiments were selected, by-passing the refrigerated separator. Operating in this manner, a selective extraction was obtained with improved global and CBG yields up to 5.3 % and 4.4 % w/w, respectively, and a CBG concentration of 83 % in the extract.
1 Introduction
Cannabis sativa L. is a plant of pharmaceutical interest that contains a wide spectrum of bioactive molecules, especially cannabinoids [1-3]. They are a class of highly lipophilic terpenophenolic compounds, obtained by the reaction between an alkyl-resorcinol with monoterpenic units [4]. Among the range of known cannabinoids, some of them can interact with CB1 and CB2 receptors of the human endocannabinoidic system involved in nervous terminals and cytokine release modulation [5-7].
Cannabigerol (CBG) is a non-psychotropic cannabinoid that shows interesting therapeutic properties [8] as neuroprotective [9-15], anticancer [16-20], antioxidant [21], anti-inflammatory [22, 23], antibacterial [24, 25], and anxiolytic [26]. However, its concentration in Cannabis is often negligible with respect to other cannabinoids, hindering its industrial application. To overcome this problem, the plant can be genetically modified to achieve larger CBG concentrations.
Different extraction techniques to recover cannabinoids from Cannabis have been tried; some of these are as follows: maceration [27-29], ultrasound-assisted extraction (UAE) [28, 30], microwave-assisted extraction (MAE) [30, 31], pressurized liquid extraction (PLE) [32], liquid and supercritical fluid extraction (SFE) [33-37]. This last process assisted by supercritical carbon dioxide (SC–CO2) is emerging as the most promising one as no toxic organic solvents, like methanol and hexane, are required, and highly concentrated extracts in phytocannabinoids can be obtained [38], and no further post-processing steps are needed. Moreover, by changing CO2 density and by modifying operating pressure and temperature, it can be possible to selectively separate different families of compounds, like essential oils, cannabinoids, and paraffinic waxes [38, 39]. In particular, the online separation of waxes is achievable using a double separation step; i.e., two separators connected in series to the extraction line. The first one is cooled down to 0 °C to allow paraffinic waxes precipitation [40], whereas the second separator works at lower pressure and temperature between 25 °C and 30 °C, to favor the collection of the extract and CO2 volatilization [39, 40]. The choice of the double separation step is convenient when looking at the conventional strategies to achieve waxes precipitation. For example, winterization consists of mixing the extract with ethanol, bringing the solution to −40 °C, and then, the precipitated matter (waxes) is removed by filtration [39]. This process has several drawbacks: First, organic solvents are needed, and an ethanolic solution of the extract is obtained; moreover, bringing the solution to very low temperatures is expensive. Therefore, the utilization of a direct and online separation step in SFE processing can overcome these problems, as demonstrated in previous works [39-41].
Generally speaking, several attempts to extract cannabinoids from Cannabis using supercritical or liquid CO2 have been carried out in the literature, although without using the fractional separation scheme [42-44]; but, to the best of our knowledge, no experiments related to the recovery of highly concentrated CBG extracts from Cannabis are reported. Indeed, as a rule, cultivars are richer in cannabidiol (CBD), and therefore, most of the studies are focused on CBD extraction. For example, Marzorati et al. [42] performed SFE on the Finola variety of a hemp cultivar working at 38 MPa and 60 °C. These authors did not work using the fractional step, and paraffinic waxes precipitated in the same separator as the rest of the extract. Consequently, although they managed to recover an extract concentrated in CBD, they needed to post-process it to eliminate waxes. Performing winterization on SFE extract at −15 °C for 36 h, followed by flash chromatography, the final product reached a CBD concentration of about 80 %. However, also in this case, an organic solvent (ethanol) was used for the winterization step, and a long post-processing was required. Karğılı and Aytaç [43] explored the effect of pressure, temperature, and the addition of ethanol as cosolvent on CBD extraction. The highest CBD yield (2.15 %) was obtained when working at 33 MPa, 40 °C and adding 2 % w/w ethanol to CO2 flow rate. After 2 h extraction at 15 MPa and 40 °C, with no cosolvent, the highest value of CBD yield to global yield ratio (43 %) was obtained. Therefore, also in this case, post-processing should be needed to further enrich the extract in CBD. Qamar et al. [44] investigated the effect of CO2 density on the extraction yield: at low densities, like 231 and 590 kg m−3, CBD yield increased from 0.81 % to 3.08 % w/v, respectively. In contrast, working at a CO2 density of 818 and 911 kg m−3, CBD yield changed from 15.6 % to 12.6 % w/v, respectively. This slight decrease in CBD yield was attributed by the authors to a more liquid-like behavior of CO2 at larger pressures that slowed the diffusion of the solvent in the vegetable matrix.
In summary, it can be stated that the choice of the operating conditions for the extraction of cannabinoids largely influences the values of yield and selectivity obtained. Moreover, the case studies reported previously did not attempt to fractionate the extract from paraffinic waxes.
As far as CBG extraction is concerned, there are only few studies mentioning its recovery from Cannabis, and they are mainly related to the utilization of conventional techniques [34, 45]. Therefore, the aim of this work is to assess for the first time the SFE process feasibility to obtain a CBG-rich extract from Cannabis. To achieve this goal, a specific CBG-rich cultivar was used, and different SFE operating conditions and plant schemes were investigated to increase CBG concentration in the extract.
2 Materials and Methods
A Cannabis cultivar, called Fibror, was kindly supplied by Veridia SpA (Città Sant'Angelo, PE, Italy). The producer declared a CBG content of about 4.5 % w/w and a tetrahydrocannabinol (THC) content lower than 0.2 % w/w. Inflorescences were gently separated by branches and leaves and were sieved between 1000 and 2000 µm. Then, they were dried in a thermostatic oven at 60 °C, overnight. Humidity content represented about 10 % of the total vegetable weight.
Hexane and methanol (high performance liquid chromatography [HPLC] purity grade) were supplied by Carlo Erba Reagenti (Cornaredo, MI, Italy). CO2 (purity 99.9 %) was bought from Morlando Group Srl (Sant'Antimo, NA, Italy). CBG standard powder (purity 99 %) was provided by Veridia SpA.
Prior to extraction experiments, ground inflorescences were decarboxylated (DBX) to convert the acid and polar form of CBG, the cannabigerolic acid (CBGA), in CBG. Decarboxylation was carried out at 105 °C for 15 min [38].
2.1 Supercritical Fluid Extraction
SFE experiments were carried out in a lab-scale high-pressure plant [41], equipped with a 300 cm3 stainless-steel extraction vessel. The desired temperature was reached using a thermostatic bath (Julabo, mod. CORIO C-B27) and heating bands located, respectively, before and around the extraction vessel. CO2 was pumped in the extraction vessel using a high-pressure membrane pump (LEWA, mod. LDB1 MD210S). A first separator, located in a refrigerating bath (Julabo, mod. F38-EH) at −10 °C, was used to obtain paraffinic waxes precipitation. A second separator, operated at 40 °C and 3.5 MPa, was used to collect the extract. Pressure was regulated in the second separator using a back-pressure valve (Tescom, mod. 26-1700) and temperature was maintained by heating bands. Pressure was adjusted using a micro-metering valve (Milli-Mite 1300 Series HOKE, Cv 0.01 in), whereas electric resistances were located right before it to mitigate the Joule–Thompson effect due to CO2 rapid expansion. CO2 flow rate was monitored on a calibrated rotameter (ASA, mod. N.5-2500, Serval 115,022): It was set at 0.85 kg h−1 for all of the extraction experiments. Temperature along extraction lines was monitored and controlled using thermocouples and PID controllers (Watlow, mod. 93), respectively; pressure was monitored using pressure gauges.
In each experiment, 30 g of DBX dried Cannabis inflorescences were loaded in the extraction vessel. All the experiments were performed at least twice to ensure process repeatability. Yield vs. time experimental points were obtained by collecting the extract in the second separator and weighing it at regular time intervals. When the first separator was connected to the line, waxes were collected at the end of the experiment, after depressurization.
For comparison purposes, hexane maceration of Cannabis inflorescences was also carried out. An amount of 5 g of inflorescences were inserted into teabag filters that were put in contact with 50 mL of hexane for 24 h at 300 rpm. To quantify CBG content in the extract, hexane was removed under vacuum using a rotary evaporator, and the residue was dissolved to obtain a 100 ppm methanolic solution to be analyzed by HPLC.
2.2 Characterization of the Extracts
HPLC was used to quantify CBG content in inflorescences and extracts. The calibration curve was obtained by using a CBG standard powder to correlate the area (A) below the CBG peak and its concentration (C). The calibration curve followed the equation A = 101.46·C. HPLC analysis was carried out using an Agilent Poroshell 120 EC-C18 2.7 µm, 3.0 × 50 mm2 column. The method used two mobile phases: The first one was 0.1 % v/v aqueous solution of formic acid, and the second one was a 0.05 % v/v methanolic solution of formic acid. The composition ratio between these two solutions was 60/40 by volume. Injection volume was 10 µL; the flow rate was set at 0.5 mL min−1.
Gas chromatography–mass spectroscopy (GC–MS, Agilent 8860 GC system coupled with 5977C GC/MSD system, Varian, Inc.) was used to obtain a composition profile of the extracts. Therefore, they were diluted in hexane to obtain a 1000 ppm concentration. The implemented GC–MS method for extracts analysis required the oven temperature at 40 °C for 6 min; then, it was set to rise to 270 °C using a thermal ramp of 2 °C min−1 and finally kept constant for further 40 min. The injector temperature was set at 280 °C.
Waxes analysis was carried out using a different thermal setup: the oven temperature was set at 120 °C for 4 min. Then, temperature was increased to 280 °C through a thermal ramp of 2 °C min−1 and then kept constant for 55 min. Helium flow rate was 1 mL min−1 for both analysis methods. Compounds identification was carried out using the Nist databank and the literature information, and their relative abundance in the analyzed sample was given as output from the instrument. HPLC and GC–MS analyses were performed in triplicate to ensure measurement reliability.
3 Results and Discussion
Before performing the extraction experiments, GC–MS traces of the DBX matrix were obtained to have a qualitative indication of the families of compounds contained in the vegetable matter. It is worth noting that the decarboxylation reaction favors the extraction process and increases cannabinoid concentration in the final extract [38]. The resulting traces are reported in Fig. 1.
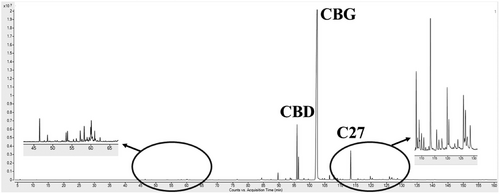
Fig. 1 indicates that the starting vegetable matter was rich in CBG as expected, and it was predominant with respect to the other families of compounds. The choice of the operating conditions should provide a CBG recovery as large as possible considering solubility data. Monoterpenes could be completely solubilized at 8 MPa and 40 °C [46], whereas sesquiterpenes show a higher affinity with SC–CO2 when working at 10 MPa and 40 °C [41, 47]. However, terpenes abundance in this matrix was relatively small. Paraffins are relatively insoluble in CO2; but they are generally co-extracted because of their location on the external surface of the vegetable matter [40]. In this case, their presence was less relevant than the cannabinoid fraction; i.e., this particular vegetable matter was paraffin-poor when compared to other matrices [46] and cannabinoids, as shown in Fig. 1.
The first part of the experimentation was carried out in traditional way to determine the amount of CBG in the Cannabis matrix and to compare it with the one provided by the supplier. The extraction was carried out on DBX inflorescences using hexane as organic solvent, as reported in Sect. 2. A 4.2 % w/w CBG yield and 8.1 % w/w global yield were obtained, with a concentration of CBG in the extract equal to about 52 %.
Once verified the coherence of the extraction values, SFE experiments were performed. Perrotin-Brunel et al. [48] investigated the effect of pressure on CBD, CBG, THC, and cannabinol (CBN) extraction, at different temperatures. Working at 42 °C and at pressures between 11 and 20 MPa, CBG solubility was always smaller than that of CBD and CBN, and larger than that of THC. Therefore, as mentioned in Sect. 1, CBD can be recovered using lower CO2 densities; e.g., working at 15 MPa and 40 °C [43], which corresponds to a CO2 density of about 0.78 g cm−3. CBG, instead, behaves differently from CBD due to its molecular structure that resembles the one of paraffinic waxes. For this reason, larger CO2 densities are needed to form a solvation complex with CBG rather than CBD. Therefore, considering solubility studies, it was chosen to carry out the extraction experiments on the DBX matrix working at 18 MPa and 40 °C, which corresponds to a CO2 density of 0.82 g cm−3.
3.1 Fractional Extraction
The first series of SFE experiments was performed using the fractional scheme; the adopted operating conditions were 18 MPa, 40 °C, and CO2 flow rate of 0.85 kg h−1. Both the first refrigerated separator and the second one were connected to the process line, in such a way that paraffinic waxes could precipitate and the extract could be collected in the second separator, as demonstrated in previous works [41, 47]. The yield vs. time experimental points obtained in these experiments are reported in Fig. 2.
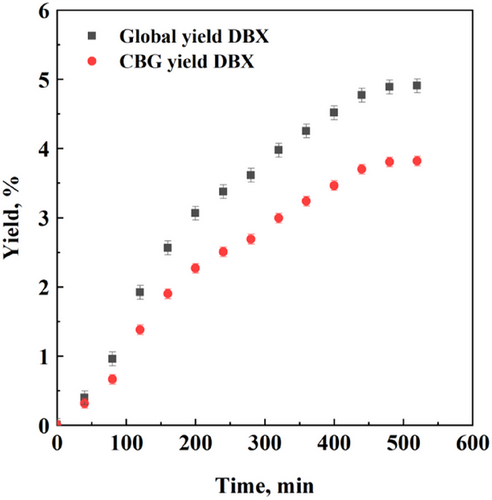
Fig. 2 shows that the experimental points follow the conventional exponential trend, approaching to an asymptotic value. The global yield stopped at about 4.8 % w/w, whereas CBG asymptote was located around 3.8 % w/w. As far as process selectivity is concerned, it can be calculated by the ratio between CBG yield and global yield: It was about 79 %. This large value was obtained without further post-processing of the extract: This is one of the main advantages of using SFE technology to recover cannabinoids, especially if looking at a biopharmaceutical application of these extracts, in which contamination has to be avoided.
However, it is worth noting that the value of CBG collected in these experiments did not correspond to the CBG content indicated in the starting matrix (about 4.5 % w/w). It means that part of CBG was not extracted or lost before the second separator. To investigate the mechanism behind this result, GC–MS traces of the extract and waxes were obtained.
GC–MS results indicated that the extract collected in the second separator was not contaminated by paraffinic waxes (Tab. 1), but part of CBG was lost in the first separator. The explanation of this phenomenon relies in the paraffin-like chemical structure of CBG; indeed, there were no other cannabinoids (e.g., CBD) precipitated in the first separator.
| Compound name | SFE, fractional scheme, 18 MPa/40 °C | |
|---|---|---|
| I separator | II separator | |
| Caryophyllene | 0 | 2.37 |
| Cis-Caryophyllene | 0 | 1.04 |
| Aciphyllene | 0 | 0.28 |
| Patchoulene | 0 | 0.22 |
| β-Selinene | 0 | 1.33 |
| α-Selinene | 0 | 2.12 |
| Caryophyllenyl alcohol | 0 | 0.43 |
| β-Guaiene | 0 | 2.45 |
| Gurjunene | 0 | 3.56 |
| Aristolene | 0 | 0.59 |
| Caryophyllene oxide | 0 | 1.52 |
| α-Farnesene | 0 | 3.19 |
| β-Eudesmol | 0 | 2.36 |
| α-Eudesmol | 0 | 0.61 |
| Selinadiol | 0 | 0.44 |
| Total terpenes | 0 | 22.72 |
| Cannabidiol | 0 | 30.21 |
| Cannabichromene | 0 | 3.96 |
| Cannabigerol | 87.10 | 42.74 |
| Cannabicyclol | 0 | 0.37 |
| Total cannabinoids | 87.10 | 77.28 |
| Heneicosane C21H44 | 2.10 | 0 |
| Tetracosane C24H50 | 0.90 | 0 |
| Heptacosane C27H56 | 4.90 | 0 |
| Nonacosane C29H60 | 2.60 | 0 |
| Triacontane C30H62 | 1.10 | 0 |
| Hentriacontane C31H64 | 1.30 | 0 |
| Total waxes | 12.90 | 0 |
To dive deeper into the indications provided by GC–MS traces, the calculation of the relative abundance of the compounds detected in the extracts and waxes was carried out. The obtained values are reported in Tab. 1.
The relative abundances among compounds in the waxes and extract confirm that the extract was rich in CBG, but part of it was collected in the first separator. For this reason, the following set of experiments was carried out without using the first separator; i.e., an SFE non-fractional scheme was adopted.
3.2 Non-Fractional Extraction
The previous results demonstrated that the adoption of SFE fractional scheme was not the right arrangement to obtain the complete collection of CBG in the extract, due to its paraffin-like structure that induced its partial precipitation in the first separator. Therefore, to improve CBG recovery, a set of experiments was carried out by disconnecting the first separator and by-passing it. All the matter extracted at 18 MPa and 40 °C was recovered in the second separator that was the only one connected to the extraction line, operated at 3.5 MPa and 40 °C. The obtained yield vs. time experimental points are reported in Fig. 3.
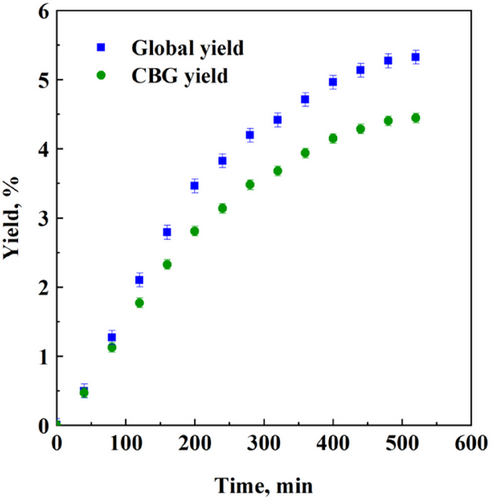
Fig. 3 highlights that, operating in this way, the maximum global and CBG yields were 5.3 % and 4.4 % w/w, respectively. Both yields increased with respect to the previous SFE fractional experiments, and the ratio between CBG yield and global yield was about 83 %.
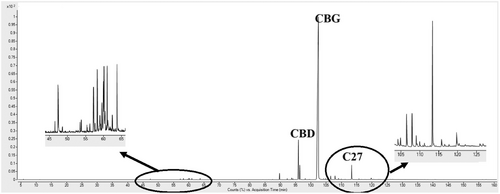
GC–MS traces and the related relative abundances of compounds extracted in the SFE non-fractional experiments are reported in Fig. 4 and Tab. 2, respectively.
| Compound name | SFE, non-fractional scheme, 18 MPa/40 °C |
|---|---|
| Extract | |
| Caryophyllene | 0.08 |
| Cis-Caryophyllene | 0.06 |
| β-Selinene | 0.06 |
| α-Selinene | 0.08 |
| Caryophyllenyl alcohol | 0.05 |
| β-Guaiene | 0.30 |
| Gurjunene | 0.39 |
| Caryophyllene oxide | 0.29 |
| α-Farnesene | 0.49 |
| β-Eudesmol | 0.38 |
| α-Eudesmol | 0.10 |
| Selinadiol | 0.14 |
| Total terpenes | 2.42 |
| Cannabidiol | 3.91 |
| Cannabichromene | 1.16 |
| Cannabigerol | 90.21 |
| Cannabicyclol | 1.14 |
| Total cannabinoids | 96.42 |
| Heneicosane C21H44 | 0.12 |
| Tetracosane C24H50 | 0.08 |
| Heptacosane C27H56 | 0.92 |
| Nonacosane C29H60 | 0.01 |
| Triacontane C30H62 | 0.01 |
| Hentriacontane C31H64 | 0.02 |
| Total waxes | 1.16 |
Looking at either Fig. 4 or Tab. 2, it is confirmed that the peaks of paraffins are not relevant with respect to the ones of cannabinoids. The percentage of waxes in the extract was limited to 1.16 %, whereas CBG relative abundance was 90.21 %. Moreover, CBG percentage was in this case larger than the one measured in SFE fractional experiments; i.e., its yield (4.4 % w/w) was larger and very similar to the yield indicated by the supplier. Overall, the choice of not fractionating the extract was correct: process selectivity and CBG recovery benefited from it. It is also very interesting to compare these results to the ones obtained by liquid extraction: that process extracted a very relevant quantity of undesired compounds, leading to an 8.1 % vs. 5.3 % w/w yield, confirming the high selectivity of SFE process.
4 Conclusions
In conclusion, the feasibility of using SFE on a CBG-rich Cannabis cultivar was assessed in this work. Extraction experiments highlighted that CBG can be properly extracted at 18 MPa and 40 °C, and the extraction plant scheme affected CBG recovery and purity. In particular, the double-step fractional setup was in this case detrimental to CBG recovery, because of the paraffin-like behavior of this cannabinoid that led to its partial precipitation in the first separator. In the single separation step, instead, CBG recovery increased up to 4.4 w/w, with a CBG concentration of about 83 % in the total extract. Therefore, the solution proposed in this work can represent a valid strategy to process CBG-rich inflorescences, exploiting the full potential of supercritical fluid technologies with a view to process intensification.
Acknowledgments
The authors thank Salvatore Gallicchio for the help provided during the experiments carried out for his Master Thesis in Chemical Engineering at the Department of Industrial Engineering of the University of Salerno, Italy.
Open access publishing facilitated by Universita degli Studi di Salerno, as part of the Wiley - CRUI-CARE agreement.




