Improving the Efficiency of a Bioreactor Equipped With Mixed-Flow Impellers
Abstract
The performance of multiple coaxial hybrid impellers in a stirred bioreactor has been experimentally characterized. To accomplish this objective, tests have been performed concerning the growth of the bacterium Azotobacter vinelandii, alginate production, sucrose consumption, and viscosity. The bacterial synthesis of the exopolysaccharide alginate results in an augmented viscosity during cultivation, thereby influencing the oxygen transfer rate. Following a comparative analysis of the results obtained from the traditional Rushton turbines, it has been observed that implementing hybrid impellers yields enhanced biomass production, increased alginate output, and the generation of more viscous cultures. These findings are substantiated by the hydrodynamic data presented in a preceding study.
1 Introduction
Alginate is a polyanionic polysaccharide that contains two monomers linearly attached, α-l-guluronic acid (G) and β-d-mannuronic acid (M), forming g or M blocks or alternative G/M. Industrially, the primary source of alginate is brown algae strains. Some bacteria, like Azotobacter and Pseudomonas, can also produce alginate. Microbial production is likely to be preferred over marine production for polymers that need specific characteristics tailored to particular industries, especially regarding their M/G ratio or molecular weight. In industrial applications, this biopolymer is extensively used as a stabilizer, thickener, and microbe-resistant coating material in food processing. Alginate has been extensively used due to its favorable applications, such as biocompatibility and easy gelation. It is a biomaterial with attractive applications in wound healing, drug delivery, and tissue engineering, among others [1].
From an industrial standpoint, alginate is commonly produced in gas–liquid systems due to its high mass and heat transfer coefficients, suitable liquid- and gas-mixing capacities, and longer gas residence times in the liquid phase. Gas–liquid systems are also known as bioreactors when they maintain a biologically active environment.
The success of a stirred tank bioreactor-based process relies mainly on maintaining well-defined and regulated culture and operating conditions, such as pH, temperature, and dissolved oxygen concentration [2]. Inadequate mixing can result in the uneven distribution of nutrients and oxygen, leading to limited growth and production [3]. On the other hand, vigorous agitation can cause high strain rates, which can damage or inhibit the growth of some sensitive bacterial cells [2, 4]. The agitation system is a fundamental element of the bioreactor because it guarantees the uniform distribution of the components within the tank. In this sense, the choice of impellers is a critical factor when designing bioreactors. The performance of a stirred tank bioreactor is highly dependent on the flow fields generated by the impellers that are used [5]. Rushton turbines (RTs) are the most used impellers, showing some advantages over other geometries in gas-liquid mixing systems [6]. Among other benefits, they require less power than other impellers. However, when working with high-viscosity media, RTs are prone to creating isolated, well-mixed regions around the impellers and dead regions in the rest of the bioreactor [7]; however, such regions tend to vanish as the agitation speed increases. There is a close relationship between the turbulent characteristics of RTs and the strain they generate, which, in limited cases, leads to sublethal cell deaths [8, 9]. The cell damage depends highly on the shear to which a particular species is subjected [10]. Axial flow impellers are proposed as a viable alternative to radial flow impellers. Although the working fluids experience moderate shear rates, certain drawbacks, including torque instabilities, necessitate high power demands during operation in the down-pumping mode. Additionally, there is a tendency to create suboptimal mixing zones near the tank's perimeter [11]. To address the deficiencies above, a mixed-flow impeller, referred to as the axial–radial impeller (ARI), has been developed and documented in other sources [12]. Compared to RTs or pitched blade turbines, the benefits of employing coaxial mixers featuring ARIs have been reported when mixing pseudoplastic fluids or fluids that exhibit yield stress [13, 14]. Shi et al. [4] proposed the design of a new impeller based on annular cage geometry to produce mammalian cells in submerged cultures. They found higher oxygen transfer rates (OTRs) in water and cell-free culture media than in traditional impellers [4]. However, the performance of mixed-flow impellers in bioreactors has been scarcely reported. Using particle image velocimetry, Posadas et al. [7] characterized the hydrodynamic performance of three coaxial mixed-flow impellers in viscous solutions of colloidal sodium alginate as a model fluid. The ARIs were compared with radial flow impellers and axial flow impellers. The results indicate that the mixed-flow impeller performs superior to alternative impellers, attributed to its ability to generate a more uniform flow distribution. This facilitates enhanced pump efficiency, improved distribution of vertical currents, and increased turbulent intensity, ultimately leading to superior agitation throughout the tank [15]. These results showed that mixed-flow impellers have high potential in fermentation processes involving shear-sensitive cultures, the viscosity of which increases during the process.
This work aims to characterize the performance of a bioreactor equipped with hybrid impellers (ARIs) while developing an authentic culture. This characterization focuses on the bacterial growth rate, alginate production, sucrose consumption, and rheological evolution using Azotobacter vinelandii cultures to produce biopolymers. A. vinelandii is recognized as possessing one of the highest oxygen demand rates among all documented microorganisms, thereby augmenting the complexity of the transport phenomena under which the current impeller's performance was evaluated. For comparison purposes, experiments have also been done with RTs, which are commonly used in this type of culture. The results obtained from this study are supported by the hydrodynamic findings reported in a previous study [7].
2 Materials and Methods
2.1 Bioreactor Setup
Biological tests were conducted on 14-L bioreactors manufactured by New Brunswick Scientific, equipped with three coaxial impellers affixed to the agitation shaft with a diameter of 0.009 m. The bioreactor consisted of a cylindrical vessel with a plane bottom. Four equally spaced baffles were placed to minimize vortexing in the free surface. On the other hand, for the gas phase, gas was supplied at the center of the tank, below the impellers, through a ring sparger having 22 holes 0.001 m in diameter. Fig. 1 shows the experimental arrangement, and Tab. 1 summarizes the experimental conditions used for this work.
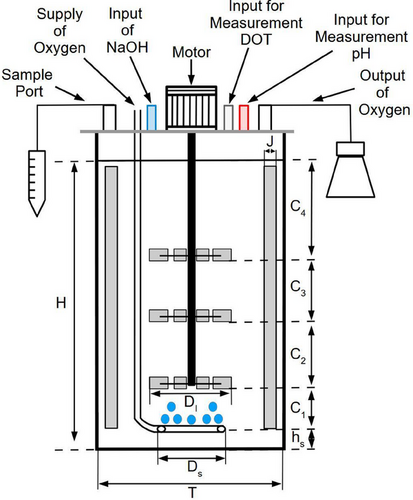
| Parameter | Value |
|---|---|
| T [m] | 0.21 |
| H [m] | 0.272 |
| hs [m] | 0.03 |
| DI [m] | 0.07 |
| Ds [m] | 0.06 |
| J [m] | 0.02 |
| C1 [m] | 0.031 |
| C2 [m] | 0.070 |
| C3 [m] | 0.070 |
| C4 [m] | 0.031 |
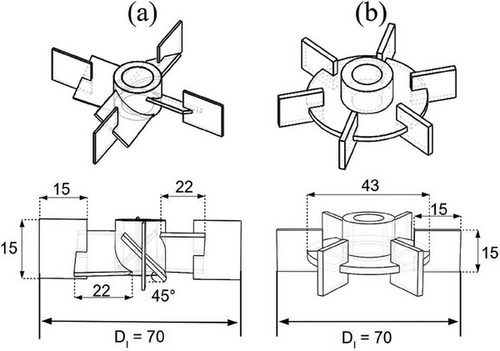
Fig. 2 shows the impellers tested in this work, which consisted of an impeller that provided mixed flow and another that provided radial flow. The ARI is an agitator that can create axial and radial flow. Its blades consist of tilted blades at 45° joined with straight blades. On the other hand, the traditional RT with six straight blades equally spaced was used as a radial flow impeller. The blades of both impellers were made with a 1.2 mm thick stainless steel sheet. Both RT and ARI rotated clockwise. Due to the above, the ARI operated in down-pumping mode.
2.2 Bacterial Strain and Medium
All cultures were carried out using the strain of A. vinelandii Lipman (ATCC9046), at least in duplicate. The strain was cultured on Burk's agar Petri dishes and stored at 4 °C monthly for immediate use [16, 17]. For prolonged times, the strain was kept lyophilized. A. vinelandii was reconstituted by growing it in 250 mL shaken flasks with 50 mL of modified Burk culture medium [16]. The modified Burk's medium was composed of (in g L−1): sucrose 20; yeast extract (Difco) 3.0, K2HPO4 0.66, KH2PO4 0.16, MOPS 1.42, CaSO4 0.05, NaCl 0.2, MgSO4 · 7H2O 0.2, Na2MoO4 · 2H2O 0.0029, FeSO4 · 7H2O 0.027. To avoid precipitation during autoclaving, the FeSO4 · 7H2O and Na2MoO4 · 2H2O solutions were prepared, separated, sterilized, and added to the sterilized bioreactor before bacterial inoculation. The pH was adjusted to 7.2 with an NaOH (2N) solution.
The pre-inocula were prepared by adding three colonies of A. vinelandii to a 500 mL flask with 0.5 mL of culture medium and incubating for 24 h at 200 rpm and 29 °C (New Brunswick Scientific Classic C25 Floor Incubator Shaker). Subsequently, 10 mL of the inoculated strain was added to a new flask containing 290 mL of the previously prepared culture medium, gauged, and incubated to be inoculated in the bioreactors.
2.3 Analytical Methods
As described previously, biomass concentrations were determined gravimetrically [16, 17]. Briefly, a 10 mL culture sample was mixed with 1 mL Na4EDTA (0.1 M) and 1 mL NaCl (1.0 M) and then centrifuged at 12 000 rpm (Eppendorf, USA). The biomass pellet was resuspended in distilled water, filtered through a Whatman filter paper, and dried at 70 °C for 24 h. The difference in weight was quantified, and the cell concentration was calculated as cell dry weight (CDW) per liter of culture. The alginate concentration was determined as described previously [16, 17]. Briefly, a 10 mL culture sample was mixed with 1 mL Na4EDTA (0.1 M) and 1 mL NaCl (1.0 M) and then centrifuged at 12 000 rpm (Eppendorf, USA) for 20 min. Cold isopropyl alcohol of 30 mL was mixed with the supernatant and shaken for 30 s. After 10 min, the resultant precipitate was filtered through a Whatman filter paper and dried at 70 °C for 24 h. Sucrose was assayed for reducing power with DNS reagent in supernatants from culture samples before the enzymatical treatment with β-fructofuranosidase (Merck-Sigma-Aldrich, USA) to hydrolyze C1 of α-glucose and C2 of β-fructose [18].
2.4 Rheological Measurement
2.5 Kolmogorov Microscale
The kinetic energy supplied by mechanical agitation in bioreactors appears in turbulent motion at larger scales, and this energy is transmitted to increasingly smaller scales until, at the smallest scales, it is dissipated by the action of viscosity in the form of heat. In this case, turbulence is modeled with eddies of different sizes. The eddies produced have the Reynolds number as their characteristic dimensionless number. If the Reynolds number of an eddy is high, the eddy will be unstable and break up, transferring energy to smaller eddies. Based on its dissipation energy, Kolmogorov theory identifies the smallest eddy in stirred systems such as bioreactors. Biological species are not affected by the eddies if they are smaller than the smallest eddies (Kolmogorov microscale) [19, 20].
2.6 Statistical Analysis
Independent samples and multiple-comparison tests (two-way analysis of variance [ANOVA] and Tukey's post hoc test) were used. A threshold significance level of 95 % (p < 0.05) was applied. All cultures were carried out at least by duplication.
3 Results and Discussion
Fig. 3 shows the behavior of A. vinelandii (ATCC9046) cultures under the two agitation systems (RTs and ARIs). These cultures were carried out at the same controlled bioreactor conditions (1 vvm of air, 29 °C, 300 rpm, and pH 7.2). The specific growth rate (µm) of biomass was calculated as the slope of a semi-logarithmic plot of biomass concentration over time during exponential growth. In this work, the specific growth rate (µm) of duplicated RT cultures (0.067 and 0.061 h−1) was almost half of that carried out using ARI impellers (0.102 and 0.118 h−1). Moreover, significant differences were observed in the maximum biomass obtained in the cultures in RTs (4.26 and 4.87 g L−1) compared to ARIs (5.99 and 5.88 g L−1). Notably, a reduction of approximately 40 % in biomass concentration was observed from 42 h to the conclusion of the culture when utilizing RT impellers. Such behavior was not detected in cultures employing ARI impellers. The elevated biomass concentrations, approaching 6 g L−1, observed in cultures utilizing the ARI impellers of the A. vinelandii wild strain ATCC9046 are infrequently documented in existing literature [21]. The decrease in biomass in bioreactor cultures during the final stages can be attributed to cell lysis but primarily to the depletion of intracellular reserve compounds related to the exhaustion of the carbon source [22]. The aforementioned reserve compounds predominantly consist of exopolysaccharides, including polyhydroxybutyrate (PHB) [23]. A. vinelandii can accumulate up to 90 % of its weight in PHB during exponential growth phases, particularly when the respiration rates of the microorganism are elevated [24]. However, at the end of the culture, when the carbon source, such as sucrose, is limited, the respiration levels of A. vinelandii decrease, and the dissolved oxygen concentration increases [22, 24]. This latter case is where A. vinelandii uses PHB as a carbon source [22, 24]. This allows the microorganism to maintain its viability, but with a significant loss in dry weight measurement (Fig. 3a), this phenomenon being more evident in the ARI impeller.
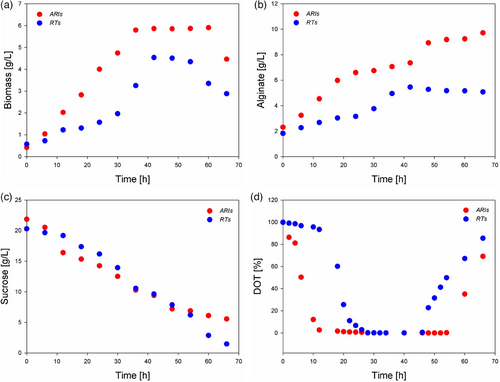
On the other hand, varying culture conditions have demonstrated differences in alginate production. For instance, Goméz-Pazarín et al. [21] showed that alterations in the filling volumes of shake flasks resulted in a variation of alginate production by A. vinelandii ATCC 9046, ranging from 1.6 to 5.8 g L−1. Maximum alginate concentrations of up to 9.0 g L−1 have been achieved only when the bioprocesses are modified from batch culture, as reported herein, to fed-batch cultures [20].
The differences observed in bacterial growth (Fig. 3a), alginate production (Fig. 3b), and carbon source consumption (Fig. 3c) must be a determinant of the mass transfer phenomena. The elevated respiratory capacity of A. vinelandii has been demonstrated to significantly influence alginate production and its molecular characteristics [25-28].
Conversely, the superior performance of ARIs can be attributed to the increased velocity fluctuations exhibited when functioning in the down-pumping mode. This phenomenon leads to a significant convergence of the flow streams from both phases, resulting in substantial velocity gradients [7]. Consequently, hybrid impellers can transfer considerable amounts of energy to the fluid, thereby producing a more homogeneous mixture and, as a result, achieving enhanced performance compared to RTs.
The oxygen uptake rate (OUR) is the rate at which a microorganism consumes oxygen. Hence, the maximum OTR can be assumed to equal the bacterial bulk's oxygen consumption rate (OUR) [29]. Here, in RTs and ARIs, the maximum OTRs can be different. However, the DOT limitation was observed (Fig. 3d).
When calculating (Yx/s) and (Yp/s) yields and using RTs, values of 0.4 and 0.33 g g−1 were calculated, respectively. In the case of ARIs, the yield values (Yx/s) and (Yp/s) were determined to be 0.42 and 0.46 g g−1, respectively. The values observed in this study are slightly higher than those documented in existing literature, which generally fall within the range of 0.10–0.25 g g−1 for both (Yx/s) and (Yp/s) [16, 17, 21, 30]. However, the comparison demonstrates the advantages of ARI impellers in this bacterial context model.
Additionally, it is understood that the microorganism's size (1.5–2.0 µm in diameter) must be comparable to the Kolmogorov microscale of turbulence to be considered affected by shear stress [37, 38]. In the bioreactor used in this study, the Kolmogorov microscale of turbulence needs to be one to two orders of magnitude higher, given that A. vinelandii was not influenced by shear stress.
Fig. 4 illustrates the temporal evolution of viscosity. After completing all rheological assessments, the samples demonstrated Newtonian behavior across the entire shear rate spectrum. Consequently, this figure presents the average value of the apparent dynamic viscosity over the culture duration, a metric that remains independent of the applied shear rate. This figure indicates that the hybrid impeller configuration yields cultures with a higher viscosity than the Rushton configuration. This result can be directly attributed to biomass production.

The kinetic and stoichiometric parameters obtained from the ARIs were compared with data reported by other researchers who utilized A. vinelandii and the RTs as the agitation mechanism. Notably, the specific growth rate associated with the RTs was analogous to the findings documented in other studies [39, 40]. In contrast, using ARIs resulted in higher product yield than values reported in previous work [41, 42].
4 Conclusions
Multiple coaxial hybrid impellers known as ARIs providing axial–radial flow have been experimentally analyzed in an agitated bioreactor using A. vinelandii as a bacterium culture commonly used to produce biopolymers. The biomass production was increased when using such impellers compared with the ones obtained with the traditional RTs. Moreover, higher alginate concentrations were obtained with hybrid impellers, resulting in more viscous cultures. These results are supported by the hydrodynamics findings reported in a previous study [7], which stated that impellers generating combined flow provide high turbulence intensity, resulting in a more homogeneous flow distribution throughout the bioreactor.
Notwithstanding the remarkable results achieved with a high oxygen consumption culture, it is imperative to investigate new microorganisms and explore novel bioproducts. This includes high cell-density bacterial and fungal cultures characterized by elevated biochemical oxygen demands, yielding products of considerable complexity, such as recombinant proteins.
Acknowledgments
David Posadas-Navarro thanks to DGAPA-UNAM Postdoctoral Program (POSDOC) for the fellowship provided. Mauricio Trujillo-Roldán thanks “Programa de Apoyos para la Superación del Personal Académico” PASPA-DGAPA-UNAM for the funds for the sabbatical leave. The authors thank Dr. Ramsés I. García-Cabrera and Dr. Adán Chávez-Castillo from the Bioprocess Unit of the Institute of Biomedical Research at UNAM. Open access funding provided by the National Autonomous University of Mexico through a transformative agreement is highly acknowledged. The funders had no role in the decision to publish or prepare the manuscript.
Open access funding provided by UNAM.
The authors have declared no conflict of interest.
Symbols used
Greek letters
-
- ε
-
- [m2 s−3]
-
- λ
-
- [m]
-
- µm
-
- [h−1]
-
-
- [m2 s−1]
-
-
- [s−1]
-
-
- [s−1]
-
- ρ
-
- [kg m−3]
-
- η
-
- [mPa s]
-
- ΔP
-
- [g]
-
- Δs
-
- [g]
-
- Δx
-
- [g]
-
- ϕ
-
- [vvm]
Sub- and Superscripts
-
- C
-
- [%]
-
- C*
-
- [%]
-
- C1
-
- [m]
-
- C2
-
- [m]
-
- C3
-
- [m]
-
- C4
-
- [m]
-
- DI
-
- [m]
-
- Ds
-
- [m]
-
- H
-
- [m]
-
- hs
-
- [m]
-
- J
-
- [m]
-
- K
-
- [mPa sn]
-
- KLa
-
- [h−1]
-
- ks
-
- [–]
-
- N
-
- [rpm]
-
- n
-
- [–]
-
- P
-
- [W]
-
- T
-
- [m]
-
- VL
-
- [m3]
-
- Yp/s
-
- [–]
-
- Yx/s
-
- [–]
Abbreviations
-
- ARI
-
- [–]
-
- ARIs
-
- [–]
-
- DOT
-
- [% v/v]
-
- OTR
-
- [–]
-
- OUR
-
- [–]
-
- Re
-
- [–]
-
- RT
-
- [–]
-
- RTs
-
- [–]
-
- vvm
-
- [min−1]
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




