Energy Requirements for Sustainable Olefin Production From CO2 via Electro- or Thermal Catalysis
Abstract
Direct electrochemical CO2 reduction as well as water electrolysis (WEL) combined with hydrogenation of CO2 to methanol (MeOH) and subsequent conversion to olefins are emerging as two possible pathways for sustainable olefin production. We provide an assessment of both routes such that they can be compared in terms of energy efficiency and projected costs. Through a sensitivity analysis, we identify bottlenecks and offer targets to achieve by catalysis design and engineers. At the current state, the electrocatalytic CO2 reduction has a much lower energy efficiency, requiring major improvements in the resulting overall cell potential and achieved faradaic efficiency. The MeOH route is mainly hampered by the overpotential required for WEL and the selectivity of olefin production, resulting in 50 kWh kg−1 of olefin.
1 Introduction
The ever more urgent climate crisis has triggered extensive research to find solutions that would make the use of fossil fuels obsolete. This transition implies the utilization of wind and solar power at an increasingly large scale, calling for the electrification of a large part of our energy intensive processes, a task especially challenging for the use of fossil fuels in the hard to abate areas such as the production of steel, aviation and shipping fuels as well as for the production of chemicals. Defossilization of the chemical sector is particularly difficult to achieve, as the industry is extremely energy intensive while also using natural gas and naphtha as the main raw materials [1]. Although the electrification of chemical processes is on the verge of being implemented in the chemical industry, the shift from, e.g., naphtha to non-fossil carbon sources such as limited biomass residues or carbon dioxide (CO2) is a longer term goal [2-6]. Although the use of recycled organic material like plastic waste helps to maintain originally fossil carbon in chemical loops (chemical recycling), utilization of CO2 is particularly intriguing as its supply from, e.g., direct air capture (DAC) is virtually unlimited providing an elegant path toward a scalable renewable chemical industry if coupled to the use of renewable electricity [7].
There are several routes from CO2 and water to bulk chemicals such as ethylene, propylene, and butylene, which account for roughly 50 % of the production of carbon containing bulk chemicals. These routes can be grouped into combined and direct synthesis pathways. Combined pathways herein are summarized as synthesis routes where green hydrogen (and oxygen) is produced through water electrolysis (WEL) and subsequently employed to hydrogenate CO2 to olefins thermocatalytically, which can include several intermediate catalytic conversion steps. The direct pathway is defined as the conversion of CO2 and water toward olefins (and oxygen) directly within the electrolyzer and without additional thermocatalytic conversions (often also called CO2 reduction reaction [CO2RR]) [8, 9].
This vision of a renewable chemical industry completely based on the educts CO2 and water (H2O), however, has one major drawback, which is the thermodynamic sink that both molecules present for carbon and hydrogen, respectively. Consequently, both routes are highly energy demanding, necessitating the utilization of gigantic amounts of electricity (estimated in Sect. 4), especially when considering that our society consumes about 180, 110, and 43 Mt year−1 of ethylene, propylene, and butylene, respectively [10-12]. Devising processes that heavily reduce the amount of energy needed as well as the associated land or water use is thus of utmost importance if these processes are to be implemented on a global scale. Fortunately, substantial progress has been made in recent years on both routes and the employed conversion steps [13-27].
2 Two Synthesis Routes for Future Olefin Production
On the backdrop of these achievements, we focus herein on comparing such a combined as well as direct synthesis pathway. Among the various possibilities for the combined pathway, we have chosen the following one: a combined electro- and thermo-chemical synthesis (comETS) where alkaline WEL-based hydrogen (H2) is reacting with CO2 using a two-step thermo-catalytic process to produce first methanol (MeOH), which is subsequently converted to olefins via methanol to olefins (MTOs) conversion [28, 29]. We chose the MTO process here over other thermal processes (e.g., Fischer-Tropsch-to-olefins) as this leads to the production of short olefins with high selectivity and is about to be implemented on a larger scale (e.g., the methanol-to-propylene [MTP] process) [25, 30]. This is compared to the direct alkaline electrocatalytic synthesis of CO2 to olefins (direct electrocatalytic synthesis [dirES]). The alkaline EL technology was chosen for WEL within comETS, as electrochemical CO2 reduction currently demands for alkaline EL, and thus a similar electrochemical technology is employed in both routes and ensures comparability of the results. The assessment is carried out from a materials/catalysis and engineering perspective, particularly focusing on the energy requirements and associated costs (both OPEX and CAPEX). With that we can compare both synthesis routes but also determine the main bottlenecks still existing and identify where technological improvements will have the largest impact and where future research efforts should be directed toward.
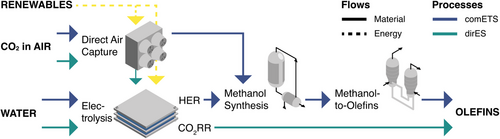
Sch. 1 shows a schematic representation of the major components of both the comETS and the dirES process route. Both start with CO2 recovery by DAC and employ the electrochemical oxygen evolution reaction (OER) at the anode of an alkaline electrolyzer, where hydroxide ions are consumed. For the comETS process, electrons and water are simply converted to hydrogen and hydroxide at the cathode (hydrogen evolution reaction [HER]; thus, the electrolyzer represents a standard alkaline water-electrolyzer). This is followed by two thermo-chemical processes that are both exothermic: The conversion of CO2 and H2 to MeOH and the subsequent MTO process with the formation of ethylene and higher olefins, mostly propylene. The last step consists of cryogenic distillation (CD). In the dirES route, water, electrons, and CO2 react at the cathode to ethylene and hydroxide (CO2RR), which is afterward worked up through acid gas removal (unconverted CO2 and water) and CD (carbon monoxide (CO) and H2 formed as by-products). Unlike in comETS ethylene is produced without the formation of propylene.
3 Assessing Energy Demand, Production Output, and Costs
3.1 Assessing Energy Demand, Production Output, and Costs
We use two approaches to assess and compare the energy demand of both process routes. First, we do this on the basis of their mass and energy balances, and second, by a cost calculation. These are described with analytical equations and solved in a spreadsheet software. For the detailed description of the calculations, see for dirES the SI chapter 1 and for comETS the SI chapter 2. Assumptions and input parameters like e.g., energy demand of DAC, faradaic efficiency (FE) toward ethylene, overpotentials, etc., were taken from literature. Where possible data closer to industrial scale and for technical operation were taken, but especially for CO2RR single cell lab data are the major source, and the uncertainty is the highest (see Supporting Information Tab. S1 for assumptions made for CO2RR and the corresponding references). As for comETS, the temperature levels are higher and heat integration is possible and needed, starting from methanol synthesis (MS) up to the final purification a flow sheet simulation with heat-integration was carried out to provide the input data for the mass and energy balances within the spreadsheet. As a result, the total energy demand in terms of kWh needed per kg olefin (ethylene in dirES, ethylene and propylene in comETS) produced can be compared but also analyzed in terms of energy demand for each individual process step shown in Sch. 1. Furthermore, we performed a sensitivity analysis to unravel the influence of key performance measures for some reaction steps (ethylene FE, overpotentials for CO2RR and OER, cathodic CO2-conversion, ohmic losses [OL], and olefin selectivity) on the overall process efficiency, thus identifying the main contributors of the energy demand. As the renewable electricity input will most likely be the limiting source for the foreseeable future, both processes are set to the same EL power input of 1 GW, with the output of olefins hence varying as they depend on the process efficiency. One gigawatt was chosen, as this scale is an often cited target from both politics and industry [31, 32]. The economic estimation is based on the factor method and is employed for all process steps displayed in Sch. 1. A detailed description of the models as well as the economic estimation are given in the SI.
3.2 Analysis of Major Contributions to Energy Demand and Costs
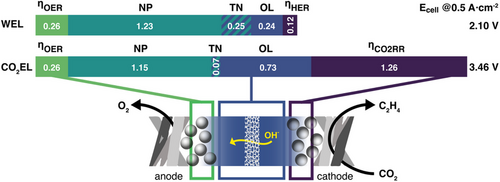
We start by analyzing the results of the comETS route in terms of energy requirements for the “current state.” Fig. 1b shows the specific energy demand for the four main parts of this process. State of the art DAC is assumed to consume 2 kWh (combined thermal and electrical power) per metric ton of CO2 captured which translates to 10 kWh kg−1 of olefins produced [33]. Obviously, for the comETS process WEL is the major driver for its huge energy consumption, requiring an energy input of 30 kWh kg−1 olefin. Our assessment divides WEL [34] into three separate contributors, being HER, OER, and OL, in order to obtain insights into the significance of these contributions. Fig. 2 shows these as electrical potentials for a single EL cell, together with the Nernst Potential (NP) and additional demand for the heat of reaction to achieve thermal neutrality (TN). In this context, thermal neutrality (or thermoneutral limit) refers to the potential at which the electrochemical reaction operates without any net heat exchange with its surroundings, marking the cell potential at which heat management requirements are minimized. It can be seen that roughly 30 % efficiency gains could be achieved when achieving zero OL and zero overpotentials. As discussed elsewhere, these losses are mainly located at the OER (3.7 kWh) and OL (3.4 kWh) [35, 36].
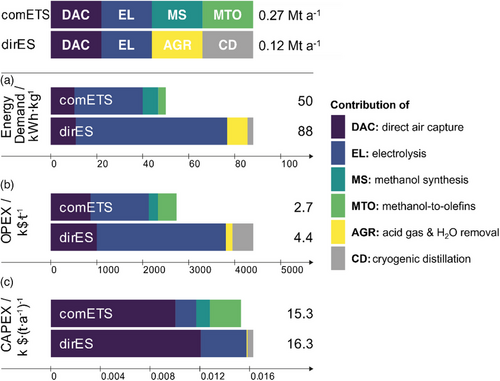
Continuing our assessment of comETS, we will now discuss MS and the MTO process. Both are highly exothermic (CO2 + 3H2 → MeOH + H2O ∆H = −49.2 kJ mol−1; 2MeOH → C2H4 + 2H2O ∆H = −134.0 kJ mol−1), thus requiring only a small additional energy input of 6.8 and 3.3 kWh kg−1 olefin for the MeOH and MTO process, respectively. Notably, the overall energy input needed for the production of 1 kg of olefin (60:40 ethylene to propylene) is 50 kWh via the comETS route. Using 1 GW of electrolyzer energy input would hence lead to a production capacity of 0.27 Mt a−1 olefins.
Although the alternative dirES process route is far from being implemented by the chemical industry, extensive research during the last decade led to significant improvements in terms of overpotential reduction and higher FE [15, 17, 37, 38]. Here, we assumed that this process would be executed at a current density of 500 mA cm−2, with an CO2RR overpotential of 1.26 V, needed to achieve an ethylene FE of 60 % (determined from data of [17, 39], using a procedure detailed in Supporting Information Sect. S1.2). Using these assumptions, we identify the electrolyzer as the main energy consumer, using 66 kWh kg−1 olefin (Fig. 1b). Based on the thermoneutral limit, the energy losses are 65 % of the total electrolyzer energy demand, giving ample room for improvement (Fig. 2). Main contributors to energy losses are the need for a gas diffusion cathode electrode resulting in higher OL, but also the high CO2RR overpotentials, which alone translate to 56 % of the energy losses. These high potentials are currently needed to gain sufficient selectivity toward ethylene within the EL. Continuing with the downstream processes in the dirES route, acid gas removal and CD add 8.8 and 2.5 kWh kg−1 ethylene, respectively. Overall, the energy demand for dirES is thus 88 kWh kg−1 ethylene, which is nearly double that of comETS. Using again a 1 GW electrolyzser setup would result in a production capacity of 0.12 Mt a−1 of olefins.
When analyzing process costs, we observe that there are distinct differences and one huge challenge. First, OPEX is extremely high (see Fig. 1c), being 2.7 and 4.4 k$ tolefin−1 for comETS and dirES, respectively. By far the main cost driver is the huge electricity demand for both processes, mainly associated with DAC and the electrolyzer. As dirES has a much higher electricity demand than comETS, its OPEX is more than 60 % higher. Note, that here we assumed electricity costs of only 4 ct kWh−1. In terms of CAPEX (Fig. 1d), both dirES and comETS are roughly similar at around 15 k$ tolefin−1. Thus, the lower absolute investment costs for dirES are counterbalanced by the lower absolute ethylene production amount and cannot be leveraged for the scenario studied. Obviously, neither of these processes would be economically competitive with olefin production from fossil resources today (currently having an OPEX of appr. 850 $ tolefin−1 [40]). However, this production is non-renewable and associated with large CO2 emissions. It is therefore anticipated that future CO2 credits make the renewable routes economically attractive.
3.3 Sensitivity Analysis and Impact of Future Improvements
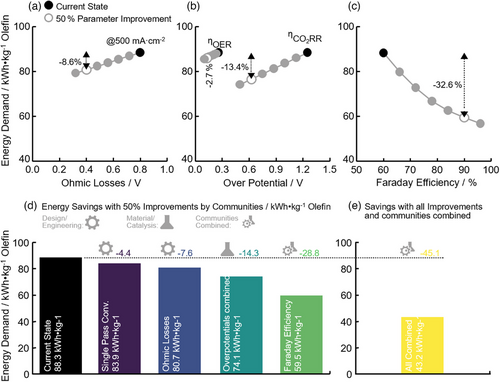
To guide research and development on CO2RR, we performed a sensitivity analysis and deduced how single improvements change the overall energy demand for the less mature dirES pathway. The single parameters studied are a reduction of the OL, reduction of the overpotential for OER and CO2RR (without lowering FE) and an increase in FE toward ethylene. Fig. 3 shows how this influences the total energy demand of CO2RR, whereas the current state refers to the assumptions used in Figs. 1 and 2. The impact of a drastic decrease for the overpotential of CO2RR, a decrease of the overpotential for OER alongside an increase of the FE toward ethylene, and solutions to tackle the large OL at high current densities can be seen in Fig. 3a–c. Engineering improvements leading to lower OL would nearly linearly reduce the energy needs (Fig. 3a) and a reduction of 50 % would lower the overall energy demand by −7.6 kWh kg−1 olefin (Fig. 3d). Reduction of the overpotentials results also in a linear response (Fig. 3b), with a 50 % reduction for OER and CO2RR resulting in savings of −4.4 and −14.3 kWh kg−1 olefin, respectively. Increasing the FE gives a non-linear response and the largest impact (Fig. 3c). In fact, a 50 % increase to a FE of 90 % would lower the energy demand by as much as −28.8 kWh kg−1 olefin. Importantly, the desired improvements have to be tackled both from a material science/catalysis but also from an engineering perspective. At the current stage, OLs can be mainly reduced through engineering approaches and improvements of the cell design [41]. Overpotentials for OER and CO2RR, on the other hand, have to be decreased through devising better catalysts [9]. Interestingly, the FE is determined by the catalyst as well as the proton vs. CO2 transport rates [14, 42, 43]. Thus, solutions increasing the FE have to be identified through combined catalyst design/engineering research. Assuming that scientists will be able to achieve these 50 % improvements in all aspects would significantly lower the total energy demand for dirES by as much as −45.1 kWh kg−1 olefin (Fig. 3e; note that the combination of all achievements leads to a lower overall reduction compared to the sum of the single improvements). The resulting demand of 43.2 kWh kg−1 olefin is similar to the more mature comETS and would, together with the largely increased absolute ethylene production rate allow to leverage the lower OPEX of dirES. We performed a similar sensitivity analysis for the comETS route with details given in the SI. Savings for this mature process are not as extensive, with the largest potential being associated with WEL and the MTO reaction (−5.3 and −9.9 kWh kg−1, respectively).
4 Conclusion on Demand Resulting for World Scale Production
The assessment of both routes shows that using CO2 and H2O as green educts in a future sustainable ethylene production requires large amounts of renewable electricity, large-scale capturing of CO2 but also extensive use of water. A production site employing the comETS process and consisting of an electrolyzer with a capacity of 1 GW (roughly 8 TWh year−1) would need the amount of 1223 kt year−1 of CO2 captured while producing 268 kt year−1 of olefins. The sites total energy demand would be 13.5 TWh, translating roughly to 2000 windmills (assuming 2.5 MW per windmill and a high capacity factor of 0.3) or an area of 130 km2 covered with solar panels (assuming a capacity factor of 0.26). This requirement nearly doubles for the dirES process as the ethylene output would only amount to 121 kt year−1 using the same 1 GW electrolyzer.
Transforming these processes to the scales with which society consumes olefins nowadays would thus necessitate almost incomprehensible amounts of electricity. Assuming a production of 290 Mt year−1 of ethylene and propylene (180 Mt/ethylene and 110 Mt year−1 propylene) and based on the current state of the technologies investigated, comETS would require roughly 2700 TWh for DAC and 8000 TWh for the electrolyzer (thus electrolyzer capacity of 1 TW), totaling 14 800 TWh of energy for the complete process, including MeOH-synthesis, MTO, and purification as summarized in Fig. 4.
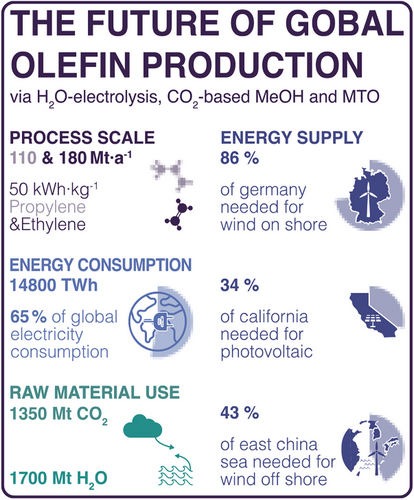
This compares to 65 % of our annual global electricity consumption and would require either an area of 86 % of Germany for wind onshore, 43 % of the east China sea (wind offshore), or 34 % of the area of California if using photovoltaics. The production would require capturing 1300 Mt CO2 per year equaling 3.53 % of energy-related CO2 emissions in 2022 worldwide [44]. (These numbers are given in the SI also for dirETS and the improved routes studied during the sensitivity analysis.) Removing such large amounts of CO2 from the atmosphere could also represent an elegant approach to sequestering this greenhouse gas, if this would be stored in polymers that are not going directly into the circular economy or disposal (e.g., lightweight car components, construction materials, etc.).
Our study also emphasizes that even in the best-case scenario, production of chemicals from CO2, H2O, and renewable electricity should only be an option after we utilized the maximum possible amount from either mechanical or chemical plastic waste recycling and biomass based routes, as these processes require only a fraction of the energy. It also shows that the hard to abate areas will require excessive investment, not least by the massive amounts of renewable electricity that needs to be installed and will likely require decades before fully put in place. Keeping the scale of consumption of our society in mind, this highlights the increasingly important role of catalysis and engineering science in making a sustainable future a reality and in bringing down costs for the products of our modern society.
Supporting Information
Supporting information for this article can be found under DOI: https://doi.org/10.1002/ceat.70034.
This section includes additional references to primary literature relevant for this research [39, 45-61].
Acknowledgments
BE acknowledges the support by the Bavarian State Ministry for Science and Arts through the Distinguished Professorship Program. MEM, ND, and FS acknowledge support by the Helmholtz association.
Open access funding enabled and organized by Projekt DEAL.
Symbols Used
-
- CAPEX
-
- [103 $ (t a−1)−1]
-
- E
-
- [V]
-
- FE
-
- [%]
-
- NP
-
- [V]
-
- OL
-
- [V]
-
- OPEX
-
- [103 $ t−1]
-
- TN
-
- [V]
Greek letters
-
- η
-
- [V]
Abbreviations
-
- AGR
-
- Acid gas and water removal
-
- CD
-
- Cryogenic distillation
-
- CO2EL
-
- Carbon dioxide electrolysis
-
- CO2RR
-
- Carbon dioxide reduction reaction
-
- comETS
-
- Combined electro- and thermochemical synthesis
-
- DAC
-
- Direct air capture
-
- dirES
-
- Direct electrocatalytic synthesis
-
- EL
-
- Electrolysis
-
- HER
-
- Hydrogen evolution reaction
-
- MeOH
-
- Methanol
-
- MS
-
- Methanol synthesis
-
- MTO
-
- Methanol-to-olefins process
-
- MTP
-
- Methanol-to-propylene process
-
- OER
-
- Oxygen evolution reaction
-
- WEL
-
- Water electrolysis
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.




