Green Highly Porous Bio-Zr-MOF/Fabric Composites for Organophosphonates Detoxification
Abstract
Current synthesis methods for metal–organic frameworks (MOFs) rely on petroleum-based linkers and toxic solvents, posing environmental risks. This study introduces a sustainable approach for synthesizing bio-MOFs composites using bio-based linkers and water. This benign method achieves a substantial Brunauer−Emmett−Teller (BET) specific surface area (SSA) using various fabrics. Importantly, when Bio-MOFs are integrated onto polypropylene (PP) pretreated using atomic layer deposition (ALD), organophosphonates hydrolysis rates improved. Furthermore, bio-MOF fabric composites show >5× faster decomposition rates than the same mass of bio-MOF in powder form. This benign synthesis process offers environmental benefits and enhances degradation kinetics, suggesting significant potential in filtration, protection, and catalysis applications.
1 Introduction
Organophosphate compounds are widely used in industrial processes and agriculture, in part because of their efficacy as chelating agents [1], flame retardants [2], and pesticides [3]. Organophosphonate nerve agents, including GD (Soman), VX (venomous agent X), and the blister agent HD (Sulfur Mustard), are also known as highly toxic chemical warfare agents (CWAs) [4] and have the capacity to pose significant environmental and health risks [5]. Consequently, developing efficient methods for their degradation and detoxification is crucial. Recent studies have demonstrated the effectiveness of Zr-based metal–organic frameworks (MOFs) in catalyzing the hydrolysis and degradation of organophosphates, attributed to their unique structural characteristics and versatile functionalities [6-8]. The high surface area (SA), accessible active sites, and tailored pore sizes of MOFs facilitate the adsorption and degradation of organophosphonate molecules [9]. Additionally, the presence of metal nodes in MOFs can act as Lewis acid sites [10], promoting hydrolysis reactions. However, producing MOFs raises environmental concerns due to the extensive use of organic solvents and petroleum-derived organic linkers, highlighting the need for sustainable synthesis approaches.
Bio-MOFs represent a porous coordination polymers (PCPs) subgroup [11]. The synthesis of bio-MOFs involves the combination of metal ions or clusters with organic ligands derived from biological sources [11], offering advantages such as sustainability, biodegradability, lower cost, and reduced toxicity compared to synthetic ligands [12]. Moreover, the properties of bio-MOFs can be tailored by selecting appropriate metal ions and ligands, making them versatile for a wide range of applications and leading to the creation of ordered crystalline structures with well-defined pores and large SAs [11, 13]. Like traditional MOF materials, the pore size of bio-MOFs can be adjusted by modifying the length and chemical properties of the linker molecules to suit specific applications such as catalysis [14-17], gas storage [18-21], drug delivery [22, 23], environmental remediation [24-26], adsorption [27-30], or biomaterials [31, 32]. Additionally, post-synthetic functionalization or modification of the organic linkers can further enhance their performance [11].
Fabric composites incorporating bio-MOFs combine the unique properties of bio-MOFs with the inherent characteristics of fabric substrates, resulting in materials with enhanced SAs, improved mechanical properties, and tailored functionalities [33]. These composites find applications in pollutant remediation [34, 35], chemical sensing [36, 37], and protective wearable clothing against toxic agents [33, 38, 39].
Zirconium-based bio-MOFs, exemplified by Zr-fumarate and MIP-202, have attracted attention due to their remarkable stability, substantial SA, and adjustable pore sizes [36, 40-42]. Furthermore, compared to microporous UiO-66-NH2, Zr-based bio-MOFs notably employ water as a solvent avoiding the need for Dimethylformamide (DMF) [43], γ-valerolactone (GVL) [38], or ethanol [44] further enhancing their sustainability and environmental friendliness. These materials exhibit significant catalytic activity, particularly in hydrolysis reactions targeting toxic compounds [36, 42, 45]. For instance, hydrolysis of diisopropyl fluorophosphate (DFP) by MIP-202 in powder form achieved a conversion of ∼21 % in 10 min and 79 % in 180 min [36]. The catalytic efficacy of bio-MOFs is attributed to their high SA, accessible active sites, and customized pore structures, which facilitate the adsorption and activation of reactant molecules. Furthermore, functional groups within bio-MOFs enhance catalytic performance by providing additional binding sites and modulating reaction kinetics.
This study investigates MIP-202(Zr) (MIP, materials from the Institute of Porous Materials in Paris) as a catalyst for the degradation of organophosphonates, including nerve agents, and its integration into wearable MOF-fabric composites. This Zr-MOF incorporates α-amino acid-based aspartate linkers and exhibits high crystallinity, as well as superior pH and hydrolytic stability. Its eco-friendly synthesis, high space-time yield, and straightforward purification procedures enhance its scalability. Although MIP-202(Zr) shares structural traits with UiO-66(Zr) MOFs, its use in degrading organophosphonates [42] and nerve agents has been limited. However, this study highlights its potential for organophosphonates detoxification, including CWA applications.
The second bio-MOF studied is Zr-fumarate MOF, which was produced by utilizing fumaric acid as a biologically derived linker, water as the solvent, and acetic acid as a modulating agent [40]. The modulation approach has been pivotal in enhancing the reproducibility and crystallinity of Zr-based MOFs, offering better control over MOF formation reactions [40]. Zr-based MOFs synthesized using this approach exhibit robust characteristics and enhanced stability, even in aqueous environments. Notably, Zr-fumarate MOF demonstrates excellent stability in water, making it suitable for various industrial and biomedical applications. Furthermore, employing a water-based synthesis approach offers advantages such as cost-effectiveness and environmental sustainability.
2 Experimental Procedures
2.1 Materials
All reagents were purchased from commercial sources and used without further purification. Zirconium (IV) chloride (ZrCl4, Alfa Aesar, ≥99.5 %), fumaric acid (C4H4O4, Sigma-Aldrich, ≥99 %), l-aspartic acid (C4H7NO4, Alfa Aesar, 99 %), acetic acid (C2H4O2, Fisher Chemical, HPLC grade), deionized water, ethanol (C2H4OH, KOPTEC), hydrochloric acid (HCl, Fisher Chemical), N-ethyl morpholine (Sigma-Aldrich, ≥97 %), and dimethyl 4-nitrophenyl phosphate (DMNP, Sigma-Aldrich).
Polymeric fibrous materials. Nonwoven polyethylene terephthalate (PET) and polypropylene (PP) were received from the Nonwovens Cooperative Research Center (NCRC), North Carolina State University. Nonwoven PP fiber mats are 0.30 mm thick, with fiber diameters ranging from 0.6 to 9.0 µm. Cotton and spandex (8 %)/polyester are commercial garments. All fabrics were used as received without further treatment.
Atomic layer deposition (ALD) was used to create thin conformal inorganic TiO2 coatings on the PP fiber mats. These samples are referred to as PP@TiO2. The TiO2 was deposited directly on the PP. The fiber coating and analysis procedures follow methods developed previously for ALD modification of polymers and textile media [6, 46]. The ALD TiO2 was deposited onto PP using a lab-made hot-wall viscous-flow vacuum reactor.
2.2 Characterizations
X-ray diffraction (XRD): The crystallographic analysis employed a Rigaku SmartLab x-ray diffractometer with Bragg−Brentano alignment and Cu Kα source. XRD was utilized to assess the crystal structure and the degree of crystallization in MOF powders and composites.
Scanning electron microscopy: Images captured with an FEI Verios 460L field-emission scanning electron microscope and variable pressure scanning electron microscope—Hitachi SU3900—were utilized for the analysis. For the first scanning electron microscopy (SEM) analysis, samples underwent gold/palladium sputtering at 11 mA for 2 min. SEM was employed to assess MOF crystal dimensions and morphology, as well as the uniformity of the coating on fibers.
N2 sorption analysis: Utilizing a Micrometrics 3Flex Physisorption system, nitrogen isotherms were gathered at liquid nitrogen temperatures (−196 °C) and analyzed with Micrometrics 3Flex software. The calculations included determining the Brunauer−Emmett−Teller (BET) SA, pore size using nonlocal density functional theory (NLDFT), micropore volume at P/P0 = 0.1 (V micro), and total pore volume at P/P0 = 0.98 (Vtot). The nitrogen isotherm shape was also employed to confirm the presence of mesopore MOF crystals and MOF-fabric composites.
2.3 Methods
Zr-fumarate MOF synthesis: ZrCl4 (0.1205 g) was dissolved in 10 mL water, 1.5 mL acetic acid (modulator), and fumaric acid (0.18 g) as linker molecule was added. For reactions at 120 °C, the reaction mixture was transferred into Teflon-capped glass vials which were put into an oven preheated to 120 °C. The reaction time was 24 h. The product was filtered and washed with deionized water, then ethanol, and dried at 75 °C. Each washing and drying step was done overnight, followed by the activation step at 85 °C in a vacuum oven overnight [40].
MIP-202(Zr) MOF synthesis: l-Aspartic acid (2.8 g) was added to deionized water (5 mL) in a 25 mL round bottom flask, then ZrCl4 (2.33 g) was added, and another 5 mL of deionized water was added to remove any powder on the wall. After the reactants were dissolved, the reaction was kept at reflux for 24 h at 120 °C. The product was filtered and washed with deionized water, then ethanol, and dried at 75 °C. Each washing and drying step was done overnight, followed by the activation step at 85 °C in a vacuum oven overnight [36].
Coating the PP with 20 nm TiO2 recipe: Deposition pressure was kept at 1.8 Torr, and the temperature was 90 °C. In a general ALD TiO2 cycle, titanium tetrachloride (TiCl4) was first dosed in the reaction chamber for 1 s, followed by 40 s of N2 purge between doses. After the TiCl4 dose and N2 purge, deionized water was dosed for 1 s with another 40 s of N2 purge. We chose 300 cycles of ALD TiO2 as a standard coating thickness onto the fiber mats resulting in 20 nm of coating thickness, as determined by a spectroscopic ellipsometer (J. A. Woollam Co., Inc.) on monitor wafers coated simultaneously in the ALD reactor.
Zr-fumarate MOF on fabric: ZrCl4 (1.205 g) was dissolved in a sonicated mixture of 30 mL water, 3 mL of modulator (acetic acid), and fumaric acid (1.8 g) as linker molecule. HCl (0.5 mL) was added, and all the previous mixture was sonicated for 10 min. After sonication, fabric was added to that mixture and sonicated for 10 min. The reaction mixture was transferred into Teflon-capped glass vials, which were preheated in the oven to 120 °C. The reaction time was 24 h. The product was filtered and washed with deionized water, then ethanol, and dried at 75 °C. Each washing and drying step was done overnight, followed by the activation step at 85 °C in a vacuum oven overnight.
Growth of MIP-202(Zr) MOF on fabric: The same powder recipe mentioned above was followed, but the fabric was added and sonicated for 10 min in the solution just before baking the mixture in the oven.
DMNP hydrolysis: Either 2.5 mg of MOF powder or 14 mg of MOF fabric composite was dispersed in 1 mL of aqueous 0.45 M N-ethylmorpholine (NEM) solution through stirring at 1100 rpm for 15 min. Subsequently, 4 µL of DMNP was added, weighed, and dispersed by shaking. The solution was continuously stirred at 1100 rpm at room temperature (25 °C). At predefined time intervals, 20 µL samples were extracted and diluted in 10 mL of 0.15 M NEM aqueous solution. The formation of product p-nitrophenoxide was monitored using a Thermo Scientific Evolution 300 UV−vis spectrophotometer at 407 nm. Utilizing DMNP hydrolysis as a characterization technique allowed for direct comparisons between powder and composite materials. The determination of half-life involved linear interpolation between points closest to 50 % conversion.
Live nerve agent degradation: Caution! CWAs are extremely toxic and should only be handled by trained personnel using appropriate safety protocols. Samples were evaluated for agent reactivity using the following protocol. Materials were activated at ∼50 °C for 2 h followed by equilibration at 50 % RH overnight. Chemical agent was dosed with ∼30 mg activated sorbent samples in a ratio of 1 µL to 10 mg (∼10 wt %). A 24 h incubation step was then performed at room temperature (25 °C). The materials were then extracted using∼1.5 mL of acetonitrile for GD tests, isopropyl alcohol for VX tests, and chloroform for HD tests, and vials were chilled in dry ice for GD and HD to quench the reaction and reduce the volatility of any residual agent. The extract was analyzed by gas chromatograph–mass spectrometer, and the % removed was calculated based on mass balance from the initial starting volume of the agent.
3 Results and Discussions
3.1 MOF Powder
For this study, Zr-fumarate and MIP-202(Zr) bio-MOF powders were synthesized following the procedure shown in Fig. 1. Reactants were placed in the autoclave or the round flask and sonicated at room temperature for at least 10 min before being heated at 120 °C for 24 h to form the MOFs. Bio-MOF-fabric composites were also formed by the same process where fabric materials were placed in the vessels with the MOF reactants before sonication.
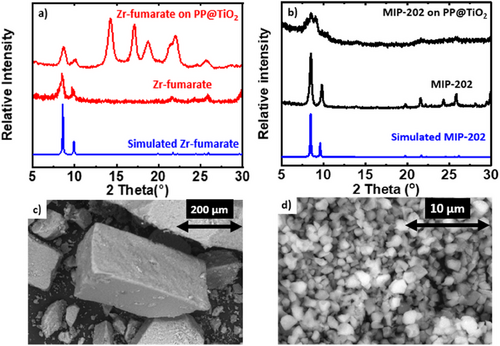
XRD patterns from the Zr-fumarate and MIP-202(Zr) powders are presented in Fig. 2. The figure also includes results from MOF-fabric composites, discussed in Sect. 3.2. Each plot includes both simulated and experimental spectra, and for each material, the experimental results correlate well with the simulated spectra. Fig. 2 also shows SEM images of the bio-MOF powders. Image analysis using ImageJ software indicates the MIP-202(Zr) particle size is ∼1 µm. The Zr-fumarate particles are substantially larger, with a typical size of ∼200 µm.
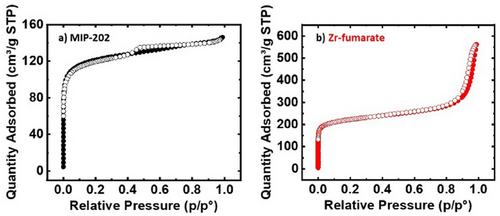
The MOFs were characterized by N2-isotherm analysis, and the results are shown in Fig. 3 and Tab. 1. The isotherm for MIP-202(Zr) displays a Type I pattern consistent with its expected microporous structure. In contrast, Zr-fumarate exhibits a Type II isotherm, indicating unrestricted monolayer–multilayer adsorption, and BET analysis indicates that the specific surface area (SSA) is 850 m2 g−1, comparable to previous reports [40]. The SSA of MIP-202(Zr) is approximately 450 m2 g−1, which represents a substantial increase, reaching up to 250 % larger than previously reported values for this MOF [37, 38]. This enhanced SA is attributed to the longer reaction time of 24 h, ensuring complete crystallization, compared to the 1 h reaction time used previously with similar initial reactant concentration [37]. Additionally, higher synthesis temperatures and reactant concentrations contribute to this increase, in contrast to lower conditions employed previously [38].
| MOF | BET [m2 g−1] | Pore width [Å] |
|---|---|---|
| Zr-fumarate | 850 | 6, 12 |
| MIP-202(Zr) | 470 | 6, 12 |
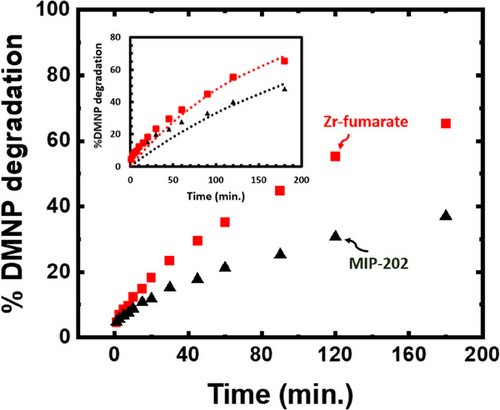
The catalytic properties of the bio-MOFs were tested by examining the rate of DMNP hydrolysis in a buffered solution (pH = 10) at room temperature (25 °C), and the results are given in Tab. 2 and Fig. 4. In Fig. 4, the experimental data are represented by the dots, whereas the dashed line in the insert illustrates the pseudo-first-order model that fits the data, consistent with findings reported in our previous publication [39]. In the DMNP degradation experiments, we standardized the catalyst weight to 2.5 mg of MOF powder, ensuring consistency and enabling a direct comparison to our earlier study involving UiO-66-NH2 synthesized with DMF [46]. The Zr-fumarate and MIP-202(Zr) demonstrate a half-life, t1/2 = 105 and 180 min, respectively. As these MOFs are constructed using the same catalytically active Zr6-based secondary building unit (SBU), the larger SA in the Zr-fumarate is expected to promote more facile access of the DMNP to the active site, thereby enabling faster degradation. The DMNP hydrolysis test was monitored at regular intervals for the first 180 min and then left for 20 h after which full conversion was confirmed. Using Zr6-based UiO-66-NH2 synthesized in our lab, degradation tests using the same conditions with the same amount of MOF catalyst showed t1/2 ∼4.8 min and a complete conversion after 60 min. The slower conversion for the MIP-202(Zr) and Zr-fumarate is ascribed to their relatively small SA and overall larger crystal size. Compared to the relatively small UiO-66-NH2 crystals (∼0.1 µm), the large MIP-202(Zr) and Zr-fumarate crystals (∼1 and ∼200 µm, respectively) can limit the DMNP mass transfer rate to the active sites within the MOF pores.
| # | Zr-fumarate | MIP-202(Zr) | UiO-66-NH2 [47] |
|---|---|---|---|
| DMNP t1/2 [min] | 105 | 180 | 4.8 |
| DMNP complete conversion [min] | <1200 | <1200 | 60 |
| Mass used in the DMNP test [mg] | 2.5 | 2.5 | 2.6 |
3.2 Bio-MOF/Fabric Composites
Bio-MOF-fabric composites were fabricated using solvothermal synthesis, incorporating Zr-fumarate or MIP-202(Zr) on PET, PE and Spandex, and PP. The fabric substrates were used in their original as-received condition. In addition, we explored bio-MOFs’ growth on PP coated with ALD TiO2.
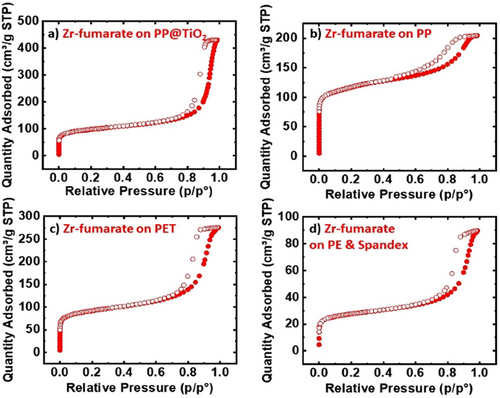
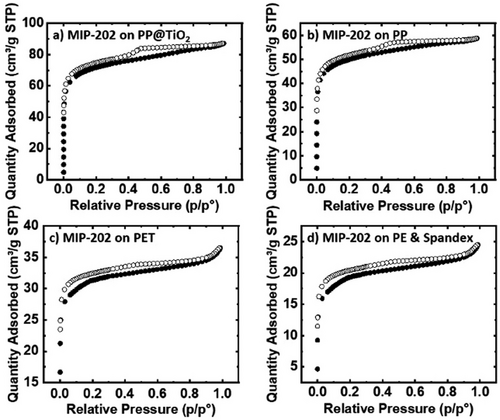
Fig. 2 shows XRD data for Zr-fumarate and MIP-202(Zr) synthesized on PP@TiO2. The peaks observed at 2ϴ > 13° correspond to the PP substrate. The alpha phase of PP, which is the most common crystalline form, is characterized by distinct diffraction peaks in XRD analysis. The prominent peaks typically occur around 2θ values of 14.0°, 17.0°, 18.5°, 21.2°, and 25.4°, corresponding to different crystallographic planes. These peaks reflect the structured arrangement of the polymer chains in the alpha form, helping to confirm its crystallinity. The PP peaks are more distinct in the Zr-fumarate on PP@TiO2 sample compared to the MIP-202(Zr) on PP@TiO2, suggesting a lower mass loading in the former. This is further confirmed by the much higher intensity of the MIP-202(Zr) peaks relative to PP, which become less visible or even disappear in the MIP-202(Zr) on PP@TiO2 sample. The MIP-202(Zr) sample shows a much greater mass loading of 650 %, compared to the 100 % mass loading of Zr-fumarate on PP@TiO2. Additionally, the broader peaks seen in the MIP-202(Zr) on PP@TiO2 sample may be due to unreacted precursors within the MOF crystals or the polymer fibers. Figs. 5 and 6 depict the isotherms of the results of MOF-fabric composites. Notably, Zr-fumarate on PP displays the highest SA, followed by Zr-fumarate on PP coated with TiO2, both exhibiting type II isotherms. Consistent with the powder samples shown above, the MIP-202(Zr) on PP@TiO2 and PP demonstrates Type I isotherms.
| # | Substrate | BET SSA [m2 gcomp−1] | MOF loading (BET) [%] | MOF Loading (mass) [%] | Adhesion |
|---|---|---|---|---|---|
| MIP-202 (Zr) | PP@TiO2 | 272 | 60 | 650 | Poor |
| PP | 191 | 65 | 64 | Poor | |
| PET | 100 | 55 | 143 | Poor | |
| PE and Spandex | 70 | 23 | 64 | Poor | |
| Cotton | N/A | ||||
| Zr-fumarate | PP | 440 | 60 | 215 | Good |
| PP@TiO2 | 360 | 45 | 100 | Good | |
| PET | 340 | 55 | 47 | Good | |
| PE and Spandex | 100 | 23 | 16 | Good | |
| Cotton | N/A | ||||
- Note: N/A indicates that the substrate polymer degraded during MOF synthesis.
For each sample, the loading value obtained from BET should be equivalent to that obtained from mass under conditions where (i) all the mass added to the fabric consists of crystalline MOF with accessible pores, and (ii) the starting fabric does not degrade or otherwise change mass during the MOF growth process. As shown in Tab. 3, for MIP-202(Zr) on PP, the two values for mass loading are similar. However, for PP@TiO2, the mass loading from the mass is larger than that from BET by a factor of ∼10, likely due to remnant unreacted MOF precursors within the composite material. This is consistent with broad peaks between 2ϴ = 8° and 10° in the XRD spectra for MIP-202(Zr) in Fig. 2. In contrast, for Zr-fumarate on PE and Spandex, the mass loading from BET exceeds that obtained from mass, consistent with the dissolution of a small amount of substrate mass during synthesis. Concerning both MIP-202 and Zr-fumarate, the fabric composites exhibited mechanical strength and flexibility comparable to regular fabrics, indicating that the synthesis process did not compromise the integrity of the fabric used. The Zr-fumarate fabric composites demonstrated better adhesion than the MIP-202 fabric composites because they had a lower presence of unreacted reactants. This was confirmed by the broad MIP-202 peaks observed in the XRD analysis and the significantly higher mass loading% from mass compared to BET.
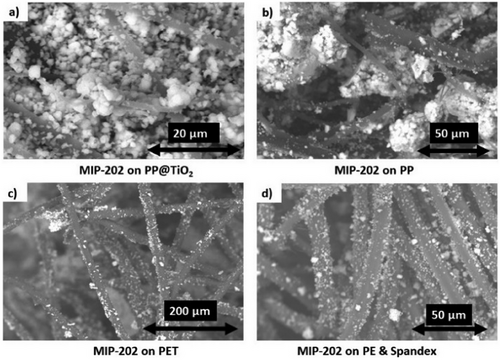
Figs. 7 and 8 display SEM images for MIP-202(Zr) and Zr-fumarate fabric composites, confirming substantial MOF growth on each fabric material. The SEM image in Fig. 7a shows that MIP-202(Zr) tended to grow on and between the fibers of PP@TiO2 substrate, consistent with the substantial MOF loading of MIP-202(Zr) on treated PP, evidenced by the high SA from the BET in Tab. 3 and the disappearance of PP peaks relative to the prominence of MIP-202(Zr) peaks in the XRD pattern shown in Fig. 2.

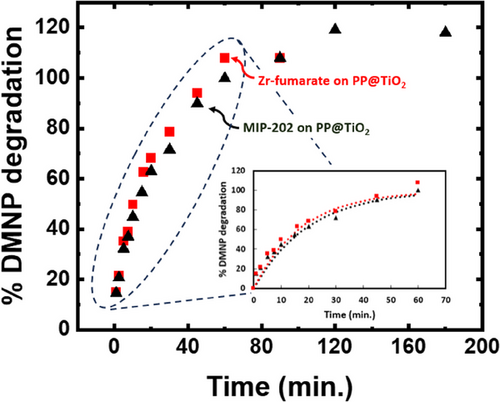
Results of DMNP hydrolysis in buffered solution (pH = 10) at room temperature (25 °C) using the MIP-202(Zr) and Zr-fumarate composites are given in Tab. 4 and Fig. 9. The experimental data in Fig. 9 are represented by the dots, whereas the dashed line in the insert illustrates the pseudo-first-order model that fits the data, consistent with findings reported in our previous publication [39]. A key goal of catalytically active fabrics is to maximize protection while minimizing the total mass of the protective garment. Therefore, normalizing to total composite mass, rather than the mass of the MOF, provides an important application-derived metric [39, 44, 46] because it allows different composites to be evaluated and compared relative to their overall mass burden. Consistent with previous studies [44, 46], the DMNP degradation experiments were performed using 14 mg of MOF-coated fabric. Using this fixed mass, we are also able to directly compare the bio-MOFs-fabrics produced here using green solvents to those synthesized previously like the UiO-66-NH2-fabrics produced using DMF or even green solvents, [44, 46] as shown in Tab. 4 and the discussion below. Under pseudo-first-order reaction conditions, the rate of hydrolysis is expected to scale with the number of accessible catalytic sites. Therefore, a faster t1/2 is expected for samples containing a larger mass fraction of accessible MOF, even when the samples have the same overall mass. In Tab. 4, the Zr-fumarate composite shows the fastest hydrolysis rate among the tested composites, consistent with the high loading of bio-MOFs onto fabrics, ensuring the attainment of a substantial SA. The implication is a rapid degradation of DMNP for the bio-MOF fabric composites, presenting a notable contrast with full DMF UiO-66-NH2 on PP@TiO2, which has an SA of 65 m2 gcomp−1. Under identical reaction conditions, this material shows t1/2 = 15 min, and complete conversion requires more than 90 min [46]. The enhanced performance of bio-MOF/fabric composites coincides with their ability to reach up to seven times greater loading for Zr-fumarate on PP compared to UiO-66-NH2 on PP@TiO2.
| # | MIP-202(Zr) on PP@TiO2 | Zr-fumarate on PP@TiO2 | UiO-66-NH2 on PP@TiO2 [46] |
|---|---|---|---|
| DMNP t1/2 [min] | 12.5 | 10 | 15 |
| DMNP complete conversion [min] | 60 | 60 | >90 |
| Mass used in DMNP test [mg] | 14.4 | 14 | 14 |
To further illustrate the advantages of bio-MOFs, a comparison is made with UiO-66-NH2 synthesized using green solvents, such as ethanol and GVL, instead of conventional full or partial DMF-based methods. A previous study from our group demonstrated that the choice of solvent and starting fabric significantly affects degradation performance. For instance, when spandex was used as the starting fabric under the same experimental conditions, the half-life for DMNP hydrolysis was 58 min for the full DMF synthesis, an average of 44 min for partial DMF, 20 min for ethanol, and an average of 29 min for GVL. These results emphasize the importance of green synthesis routes and substrate selection in improving degradation kinetics [44].
In addition to their superior performance, bio-MOFs offer significant environmental advantages due to the use of green solvents and bio-based linkers, which reduce the environmental footprint of synthesis compared to conventional MOFs. Our current bio-MOF composites not only outperform previously reported green methods in terms of degradation rates but also achieve higher mass loading while maintaining practical usability. By using the composite's total mass as an “application-derived metric,” as previously demonstrated in the literature [39, 44, 46], we provide a robust framework for evaluating materials in real-world contexts. This approach directly aligns with practical needs, such as reducing the user's burden while maximizing catalytic efficiency. The dual focus on performance and sustainability underscores the potential of bio-MOF composites for field-deployable applications, such as protective clothing or filtration systems, while also offering an eco-friendly solution for chemical degradation challenges.
Following the hydrolysis tests on simulants, further experiments were conducted to assess the solid-state degradation of live chemical agents. Tab. 5 presents the results of degradation experiments for GD, VX, and HD using Zr-fumarate and MIP-202(Zr) on PP@TiO2. Due to the extreme toxicity of these live agents, experiments were conducted only once for each sample, and reported values are considered approximate. Despite this, the results indicate that MIP-202(Zr) on PP@TiO2 achieves nearly 67 % conversion of GD in 24 h, with significantly lower degradation observed for VX and HD. Similarly, Zr-fumarate on PP@TiO2 exhibits approximately 45 % degradation for GD, 42 % for VX, and substantially less degradation for HD.
| # | MIP-202(Zr) on PP@TiO2 [%] | Zr-fumarate on PP@TiO2 [%] |
|---|---|---|
| GD degradation in 24 h | 67 | 45 |
| VX degradation in 24 h | 9 | 42 |
| HD degradation in 24 h | 0 | 5 |
The observed high percentages of GD degradation for MIP-202(Zr) and Zr-fumarate on PP@TiO2 can be attributed primarily to their elevated SA, indicating a significant number of accessible active sites [47].
In contrast, Zr-fumarate on PP@TiO2 demonstrated a higher VX degradation percentage compared to MIP-202(Zr) on PP@TiO2. VX hydrolysis typically yields ethyl methylphosphonate (EMPA) and diisopropylaminoethanethiol (DESH) [48]. We hypothesize that the distribution of hydrolysis products depends on the MOF-composite structure, and the accelerated hydrolysis of VX in the Zr-fumarate on PP@TiO2 composite may be due to the generation of EMPA, which is also known to act as a catalyst to expedite VX hydrolysis [49].
Regarding HD degradation, both MIP-202(Zr) and Zr-fumarate exhibited similar, relatively low degradation rates. The relatively slow degradation for HD was ascribed to a difference in reactant transport to the active catalytic sites. During testing, GD was observed to infiltrate and wet the fabric more readily compared to HD and VX. Ambient water present in the testing chamber can also adsorb onto the surface and facilitate the transportation of partially soluble GD, whereas the transport of less soluble HD is not significantly influenced.
3.3 Comparison between MIP-202(Zr) Powder and MOF-Fabric Composite
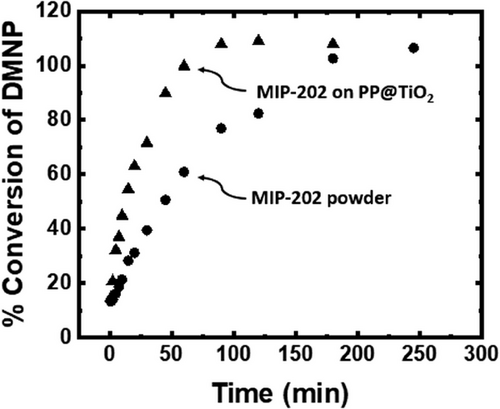
The DMNP hydrolysis in buffered solution (pH = 10) outcomes for MIP-202(Zr) on PP@TiO2, along with an equivalent mass of MIP-202(Zr) in its composite form as a powder, are detailed in Tab. 6 and Fig. 10. Remarkably, the MIP-202(Zr) fabric composite, boasting a BET mass loading of 60 %, exhibits a more rapid DMNP hydrolysis rate compared to the MIP-202(Zr) powder with an equivalent mass loaded onto the substrate. This discrepancy can be attributed to the smaller crystal size of the MIP-202(Zr) bio-MOF that undergoes heterogeneous growth on the fabric, as opposed to the homogeneous growth of MIP-202(Zr) bio-MOF, as observed in the SEM images. Consequently, the superior DMNP degradation observed in these composites highlights the advantage over utilizing the same quantity of MOF in powder form.
| # | MIP-202(Zr) on PP@TiO2 | MIP-202 as powder |
|---|---|---|
| DMNP t1/2 [min] | 12.5 | 60 |
| DMNP complete conversion [min] | 60 | 180 |
| Mass used in DMNP test [mg] | 14 | 8.6 |
4 Conclusion
We demonstrate the synthesis of catalytically active bio-MOF-fabric composites using sustainable processing methods. Using bio-based organic linkers and water as a solvent for MIP-202(Zr) and Zr-fumarate synthesis proves eco-friendly and more cost-effective than traditional petroleum-derived materials and solvents like those used for UiO-66-NH2. These bio-MOF-fabric composites are not only more applicable but also highlight the superior efficiency of heterogeneously nucleated MIP-202 in hydrolyzing organophosphonates compared to homogeneously nucleated MIP-202. The eco-friendly synthesis method allows the growth of bio-MOFs on various fabrics, achieving a BET SSA of up to 360 m2 gcomp−1 for Zr-fumarate and 272 m2 gcomp−1 for MIP-202 on PP coated with TiO2 using ALD. Even using untreated PP, a substantial SSA of 440 m2 gcomp−1 for Zr-fumarate and 191 m2 gcomp−1 for MIP-202 is attained. Importantly, the Zr-fumarate and MIP-202 bio-MOF-treated PP composites show an enhanced rate for catalytic hydrolysis of DMNP with a half-life, t1/2 = 10 and 12.5 min, respectively, compared UiO-66-NH2-composites which show t1/2 = 15 min measured under identical conditions, emphasizing the importance of high MOF loading, crystal size, and precursor concentrations in accelerating hydrolysis kinetics. Zr-fumarate on PP@TiO2 shows potential in degrading VX and GD. Overall, the use of bio-based linkers offers cost-effectiveness and environmental benefits, whereas benign synthesis processes enhance organophosphate degradation kinetics, indicating potential in diverse applications such as filtration, protection, and catalysis.
Acknowledgments
The research was sponsored by the Army Research Office and Defense Threat Reduction Agency under Cooperative Agreement Number W911NF-19-2-0154 and CB11222, respectively. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Army Research Office or the US Government. The US Government is authorized to reproduce and distribute reprints for Government purposes notwithstanding any copyright notation herein. This work was performed in part at the Analytical Instrumentation Facility (AIF) at North Carolina State University, which is supported by the State of North Carolina and the National Science Foundation (award number ECCS-2025064). The AIF is a member of the North Carolina Research Triangle Nanotechnology Network (RTNN), a site in the National Nanotechnology Coordinated Infrastructure (NNCI). MOA thanks Sarah E. Morgan for helpful training in the laboratory, Christopher Oldham for his assistance with the manuscript, and Carlotta Contreras Sepulveda for her assistance in creating the graphical abstract and Fig. 1.
The authors declare no conflicts of interest.
Author Contributions
Mai O. Abdelmigeed: Methodology, investigation, analysis, writing—original draft. John J. Mahle: Nerve agents degradation tests, writing—review and editing. Gregory W. Peterson: Writing—review and editing. Gregory N. Parsons: Writing—review and editing, supervision. All authors have approved the final version of the manuscript.
Symbols used
-
- ALD
-
- atomic layer deposition
-
- BET
-
- Brunauer–Emmett–Teller (method for measuring surface area of materials)
-
- Bio-MOF
-
- biologically derived metal–organic framework
-
- CWAs
-
- chemical warfare agents
-
- DESH
-
- diisopropylaminoethanethiol
-
- DFP
-
- diisopropylfluorophosphate
-
- DMF
-
- dimethylformamide
-
- DMNP
-
- dimethyl 4-nitrophenyl phosphate
-
- EMPA
-
- ethyl methylphosphonate
-
- GD
-
- Soman (a toxic chemical warfare agent)
-
- GVL
-
- gamma-valerolactone
-
- HD
-
- sulfur mustard (a chemical warfare agent)
-
- MIP-202
-
- materials from the Institute of Porous Materials in Paris. A type of Zr-based metal–organic framework (MOF)
-
- N/A
-
- indicates that the substrate polymer degraded during MOF synthesis
-
- NEM
-
- N-ethylmorpholine
-
- PCPs
-
- porous coordination polymers (synonym for MOFs)
-
- PE
-
- polyethylene
-
- PET
-
- polyethylene terephthalate
-
- PP
-
- polypropylene
-
- SSA
-
- specific surface area
-
- SEM
-
- scanning electron microscopy
-
- UiO-66-NH2
-
- materials from the institute of the University in Oslo. An amino-functionalized variant of the UiO-66 zirconium-based MOF
-
- VX
-
- a lethal nerve agent
-
- XRD
-
- x-ray diffraction
-
- Zr
-
- zirconium
Open Research
Data Availability Statement
The data that support the findings of this study are available within the article.




