Complete blood and urine paraprotein tests as response assessments in multiple myeloma patients treated with bortezomib, cyclophosphamide, and dexamethasone
Abstract
Background
This study assessed the effect of standardized efficacy markers on prognosis in patients with newly diagnosed multiple myeloma (MM) during the induction phase of treatment with bortezomib, cyclophosphamide, and dexamethasone (BCD).
Methods
We retrospectively analyzed clinical data in 197 newly diagnosed MM patients treated with BCD as front-line regimen at Peking Union Medical College Hospital from January 1, 2013 to December 31, 2018.
Results
There were 107 patients with International Staging System (ISS) III and 51 with paraprotein of light chain. Of these, 77 completed nine cycles of the BCD regimen. As the number of treatment cycles increased, the proportions of serum and urine immunofixation electrophoresis (IFE) tests elevated from 40.39% to 62.22% and 16.75% to 37.78%, respectively. More than 90% of intact immunoglobulin chain MM patients were evaluated for blood M protein per cycle, but that of urinary M protein was less than 60%. The detection rate of urinary M protein in light chain MM was more than 70% per cycle. Patients with a very good partial response (VGPR) had longer progression-free survival (PFS) than those with uncertain VGPR (32 vs. 26 months, p = 0.0336). Of the 141 patients who completed at least four cycles without undergoing autologous hematopoietic stem cell transplantation, those who were regularly assessed at every other cycle showed more favorable PFS than those who visited irregularly (27 vs. 22 months, p = 0.059).
Conclusion
Urinary M protein detection rate is significantly lower than that in serum, leading to an overestimation of efficacy, premature reduction of treatment intensity, and shortened PFS. Precise response assessments are critical to treatment decisions and clinical diagnoses.
Key points
-
This study evaluates the efficacy of paraprotein tests in assessing the prognosis of multiple myeloma patients treated with bortezomib, cyclophosphamide, and dexamethasone.
-
Low detection rate of urinary M protein leading to an overestimation of efficacy, premature reduction of treatment intensity, and shortened progression-free survival.
-
Precise response assessments are critical to treatment decisions and clinical outcome.
1 INTRODUCTION
Multiple myeloma (MM) is a malignant plasma cell dyscrasia with an incidence rate of 4.7 per 100,000 people.1 In the past two decades, significant treatment progress has been made, and the overall response rate (ORR), progression-free survival (PFS), and overall survival (OS) have remarkably improved.2 This is due to the widespread application of new immunomodulatory drugs such as lenalidomide, proteasome inhibitors including bortezomib, carfilzomib, and ixazomib, as well as autologous hematopoietic stem cell transplantation (ASCT).3-6
A three-drug regimen that includes bortezomib, cyclophosphamide, and dexamethasone (BCD) is one of the recommended first-line treatments for newly diagnosed MM. Clinical trials have been conducted on this regimen, but they have been highly selective, with good performance status as one of the criteria for eligibility.7, 8 Therefore, the extent to which the study populations represent real-world patients is unclear. Similarly, physicians lack data describing response assessments in these patients.
In this study, recent data from our single center were reviewed to provide a real-world perspective on the response assessment of patients with MM treated with the BCD regimen. We anticipate that our results would influence survival outcomes and guide decision-making in routine clinical practice.
2 METHODS
2.1 Patients and methods
We identified all patients with newly diagnosed MM at the Peking Union Medical College Hospital between January 1, 2013 and December 31, 2018. The BCD regimen was used as a first-line treatment in these patients. Patients were excluded if they failed to complete at least one course of chemotherapy. Response-related data such as serum protein electrophoresis (SPE), serum and urine immunofixation electrophoresis (sIFE and uIFE, respectively), serum immunoglobulin level and free light chain (sFLC), quantification of 24-h urine light chain (24hULC), bone marrow morphology, and fluorescence in situ hybridization were collected.
All patients were diagnosed according to the International Myeloma Working Group (IMWG) diagnostic criteria.9 Randomization was stratified according to International Staging System (ISS) disease stage.10 Flow cytometry was performed to confirm plasma cell clonality. Response and progression were determined according to the consensus criteria of the IMWG. PFS was defined as the time from diagnosis to initial disease progression, death due to any cause, or the last follow-up, whichever occurred first.
2.2 Follow-up
All patients were followed up either by phone or at the outpatient clinic at least once a month. Response-related data(sIFE, uIFE, sFLC, and 24hULC)were collected.
2.3 Statistical analysis
Kaplan–Meier analysis was used to estimate patient survival, and the differences between the curves were examined for statistical significance using the two-tailed log-rank test. Multivariate Cox regression analyses were performed to adjust for potential confounders. Variables with a p-value < 0.1 in the univariate analysis were included in the multivariate model. Statistical significance was set at p < 0.05. We used the statistical and graphing software SPSS 25.0 (SPSS Inc.) and GraphPad Prism 8 (GraphPad Software).
3 RESULTS
3.1 Baseline characteristics
A total of 197 patients, 53.8% males (n = 106) and 46.2% females (n = 91), were included in this study. Baseline clinical characteristics are shown in Table 1. The median age was 62 years (range: 33–79 years), and 54.3% (n = 107) had an International Staging System (ISS) phase of III. Overall, there were 160 cases of anemia, 21 cases of hypercalcemia, and 52 cases of renal insufficiency. Only 12 patients discontinued the BCD regimen after one course of treatment, and more than half of the included patients completed ≥7 cycles (Table 2). The reasons for termination of induction chemotherapy included ASCT, alternative regimens due to serious adverse drug reactions, progressive disease, or transfer to the maintenance period.
| Variables | n (%)/median (IQR) (n = 197) |
|---|---|
| Sex | |
| Male | 106 (53.8) |
| Female | 91 (46.2) |
| Age (years) | 62 (33–79) |
| Paraprotein type | |
| IgA | 42 (21.3) |
| IgG | 89 (45.2) |
| IgD | 15 (7.6) |
| Light chain | 51 (25.9) |
| International staging system | |
| I | 43 (21.8) |
| II | 47 (23.9) |
| III | 107 (54.3) |
| Hemoglobin (g/L) | 96.0 (47–156) |
| LDH (U/L) | 197.4 (75–949) |
| Creatinine (µmol/L) | 179.4 (41–1370) |
| Anemia | 160 (81.2) |
| Hypercalcemia | 21 (10.7) |
| Renal insufficiency | 52 (26.4) |
| Patients receiving ASCT | 17 (8.6) |
- Abbreviations: ASCT, autologous stem cell transplantation; IgA, Immunoglobulin A; IgG, Immunoglobulin G; IgD, Immunoglobulin D; LDH, lactate dehydrogenase.
| Cycles | n (%) |
|---|---|
| 1 | 197 (100) |
| 2 | 185 (93.9) |
| 3 | 169 (85.8) |
| 4 | 154 (78.2) |
| 5 | 143 (72.6) |
| 6 | 125 (63.5) |
| 7 | 105 (53.3) |
| 8 | 93 (47.2) |
| 9 | 77 (39.1) |
3.2 Paraprotein-related examinations
The paraprotein-related examinations are listed in Table 3. According to the results, evaluating patients' serum M protein levels was important since more than 90% underwent SPE or sIFE after each cycle. However, there was a gap in testing rates between 24hULC and serum M protein. In terms of urinary examination, less than 60% of the patients underwent 24hULC testing, compared to the 40% that underwent uIFE. As the number of treatment courses increased, the quantitative examinations (SPE and 24hULC) decreased, whereas qualitative ones (sIFE and uIFE) increased. Since sFLC is not routinely performed, an increasing trend was noted with each cycle from 39.09% after Cycle 1 to 63.89% after Cycle 9. However, only 50%–60% of the patients underwent both serum and urine paraprotein examinations, either quantitatively or qualitatively, after each cycle.
| Cycles | SPE (%) | sIFE (%) | 24hULC (%) | uIFE (%) | sFLC (%) | Both serum and urine M protein (%) |
|---|---|---|---|---|---|---|
| 1 | 68.47 | 40.39 | 58.13 | 16.75 | 39.09 | 61.93 |
| 2 | 66.28 | 36.63 | 58.14 | 19.19 | 40.54 | 56.40 |
| 3 | 65.99 | 48.98 | 55.78 | 19.05 | 48.50 | 59.59 |
| 4 | 60.16 | 51.56 | 53.91 | 19.53 | 51.30 | 61.72 |
| 5 | 56.07 | 58.88 | 42.99 | 22.43 | 48.95 | 50.85 |
| 6 | 57.95 | 59.09 | 46.59 | 21.59 | 44.00 | 55.06 |
| 7 | 53.73 | 58.21 | 41.79 | 26.87 | 56.19 | 54.41 |
| 8 | 58.62 | 63.79 | 41.38 | 29.31 | 67.74 | 57.39 |
| 9 | 57.78 | 62.22 | 48.89 | 37.78 | 63.89 | 63.83 |
- Abbreviations: sFLC, serum free light chain; sIFE, serum immunofixation electrophoresis; SPE, serum protein electrophoresis; 24hULC, 24-h urine light chain; uIFE, urine immunofixation electrophoresis.
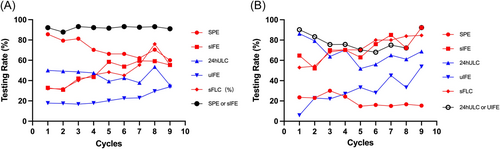
Since urine tests were performed more frequently in patients with light chain MM (LCMM) than those with intact immunoglobulin MM (IIMM), the completion rates of the five main laboratory examinations were compared between the two groups (n = 51 and n = 146, respectively) (Figure 1). More than 60% of the patients with LCMM achieved negative sIFE after the first cycle of the BCD regimen. Therefore, the proportion of SPE decreased during follow-up and was much lower than that of patients with IIMM. In most patients with LCMM, sIFE was used as a substitute for SPE. More than 50% of the patients with LCMM underwent 24hULC testing, compared to less than 50% of the patients with IIMM.
3.3 Response assessment
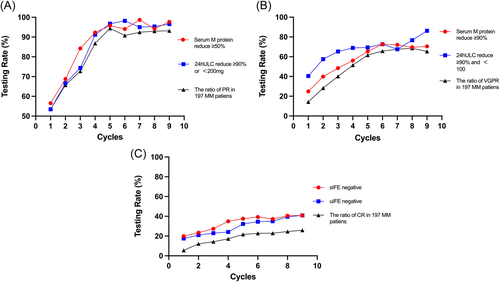
According to the IMWG criteria, partial remission (PR) required a reduction of serum M protein by more than 50% and 24-h urine M protein by more than 90% or an absolute value less than 200 mg/24 h. Thus, either serum or urine M protein assessment may overestimate the response efficacy. As shown in Figure 2, PR, very good partial response (VGPR), and complete remission (CR) rates after each cycle were depicted according to serum only, urine only, and both serum and urine tests. At most time points, real clinical responses were inferior to those evaluated using either serum or urine testing. However, not all main examinations were completed in real-world practice considering cost, convenience, and patient compliance (Table 3). Therefore, either the serum or urine examination for paraproteins overestimated the response to treatment.
3.4 Survival
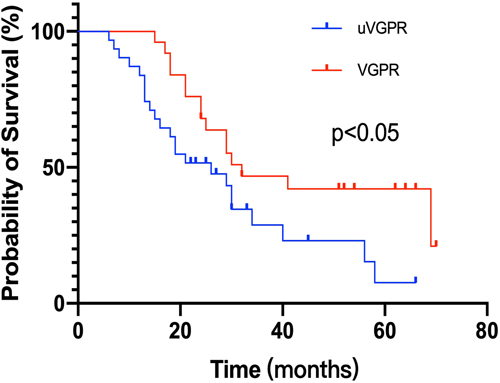
All patients with IIMM were selected to analyze the impact of response assessment on survival. We defined patients with missing urine tests but with a serum M protein reduction of more than 90% as an uncertain VGPR (uVGPR, n = 18). Compared to those who underwent both urine and serum examinations and achieved real VGPR (n = 17), patients with uVGPR showed inferior PFS (26 vs. 32 months, p = 0.0332) (Figure 3). These patients had comparable clinical and cytogenetic characteristics (Table 4). However, patients with IIMM achieving real CR or PR had similar PFS to those who only fulfilled the serum CR or serum PR criteria, respectively.
| Variables | uVGPR (n = 18) | VGPR (n = 17) |
|---|---|---|
| Female | 10 (55.6) | 9 (52.9) |
| Median age (years) | 61 (51–69) | 58 (48–69) |
| International staging system | ||
| I | 5 (27.8) | 6 (35.3) |
| II | 2 (11.1) | 2 (11.8) |
| III | 11 (61.1) | 9 (52.9) |
| Cytogenetic abnormalities | ||
| 1q21+ | 5 (27.8) | 5 (29.4) |
| 17p− | 3 (16.7) | 2 (11.8) |
- Note: Data are presented as n (%) or median (IQR).
- Abbreviations: IQR, interquartile range; uVGPR, uncertain very good partial response; VGPR, Very good partial response.
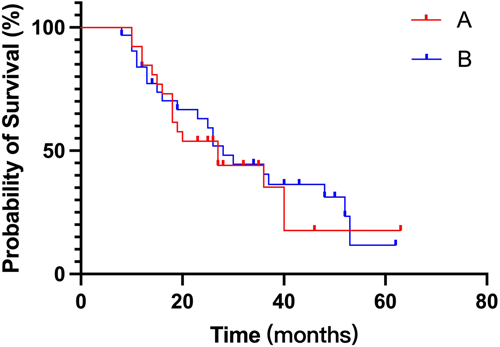
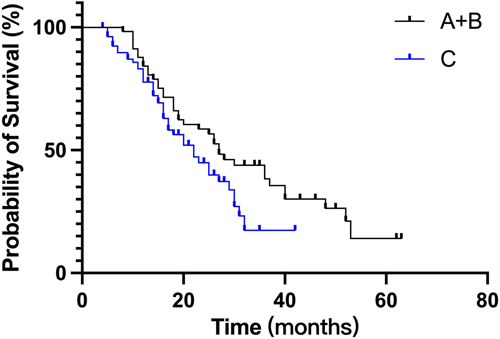
To analyze the appropriate frequency of the response assessment, 141 patients who completed at least four cycles and did not undergo ASCT were selected. Those who were regularly assessed were evaluated every cycle (Group A, n = 26) or every other cycle (Group B, n = 32). The rest were classified into the irregular assessment group (Group C, n = 83), as shown in Table 5. Interestingly, PFS between Groups A and B was quite similar (Figure 4). Patients who were regularly assessed (Group A + B) showed more favorable PFS than those who were irregularly assessed (Figure 5, 27 vs. 22 months, p = 0.059).
| Variables | Regular assessment | Irregular assessment | |
|---|---|---|---|
| A: Once a cycle (n = 26) | B: Every other cycle (n = 32) | C(n = 83) | |
| Female | 12 (46.2) | 13 (40.6) | 39 (47.0) |
| Median age (years) | 66 (51–76) | 64 (45–69) | 62 (38–79) |
| International staging system | |||
| I | 4 (15.4) | 5 (15.6) | 23 (27.7) |
| II | 6 (23.1) | 13 (40.6) | 16 (19.3) |
| III | 16 (61.5) | 14 (43.75) | 44 (53.0) |
| Type | |||
| Intact immunoglobulin | 19 (73.1) | 23 (71.9) | 64 (77.1) |
| Light chain | 7 (26.9) | 9 (28.1) | 19 (22.9) |
| Cytogenetic abnormalities | |||
| 1q21+ | 9/23 (39.1) | 10/26 (38.5) | 25/69 (36.2) |
| 17p− | 4/23 (17.4) | 3/26 (11.6) | 9/69 (13.0) |
- Note: Data are presented as n (%) or median (IQR). Group A underwent blood and urine M-protein evaluation every month. Group B underwent blood and urine M-protein evaluation at least every 2 months. Group C is neither Group A nor Group B.
- Abbreviation: IQR, interquartile range.
4 DISCUSSION
Although the clinical outcome of patients with MM has significantly improved during the last two decades, it is still a largely incurable disease. Regardless of the therapeutic regimen, there is considerable evidence that the degree of response is highly correlated with patient outcomes and is an important aspect of long-term survival.11-13 Therefore, standardized response evaluations are essential to examine the efficacy of chemotherapeutic regimens, the timing of therapy change, and other treatment factors. However, in real-world practice, due to the complexity of response criteria and the inaccessibility of special tests, urinary and serum paraprotein evaluation are largely heterogeneous. Even in a tertiary care hospital such as Peking Union Medical Hospital, complete response tests with both blood and urine samples after each chemotherapeutic course were only performed in approximately two-thirds of patients with newly diagnosed MM. The proportion of urine tests was less than 50% in patients with IIMM, while SPE was only performed in approximately 20% of those with LCMM.
Blood and urine response tests are crucial for patients with MM. Previous studies14 have shown that in patients with positive serum and urinary M protein at diagnosis, sIFE negativity is accompanied by a negative uIFE in 98.2% of the patients. In addition, patients who met the criteria for CR without uIFE availability showed minimal residual disease (MRD) and 2-year PFS rates similar to those with true CR, whereas these rates were inferior in patients achieving VGPR.15 Therefore, in patients with exclusive serum M protein positivity at diagnosis, uIFE is not necessary for establishing CR. However, according to our results, the maximum outcome cannot reach CR in most patients, and its rate in bortezomib-based regimens is less than 40%.16 In addition, our results indicated that urinary M protein evaluation is necessary to establish VGPR. Therefore, in clinical practice, serum and urinary M proteins should be simultaneously evaluated in patients with MM. A study17 that compared the performance of serum and urine measurements (72κ, 41λ) in 113 patients newly diagnosed with LCMM enrolled in the Intergroupe Francophone du Myélome 2009 trial concluded that sFLC increased the sensitivity and prognostic value compared to urinary measurements and recommended it for monitoring these patients. However, there is no clear report regarding the replacement of urine M protein evaluation in patients with IIMM. According to our results, the proportion of urine M protein tests is low because many hospitals lack these tests, indicating that samples have to be sent to commercial laboratory companies. Therefore, it is crucial to standardize serum and urine response tests in clinical practice.
Another important finding to be addressed is the frequency of M protein evaluation. MM is a chronic hematological malignancy, and regular follow-up translates to good compliance. In addition, physicians should understand patients' conditions so that they can make reasonable decisions regarding treatment. However, in real practice, the cost and complexity of response examinations greatly impair compliance by these patients. Based on our data, complete M protein testing after each treatment cycle seems unnecessary since patients undergoing examinations at every other cycle had similar survival rates as those with more frequent testing.
In conclusion, patients with MM have practical difficulties in undergoing complete paraprotein examination, such as 24-h urine collection, regular follow-up for out-of-town patients, and the availability of urine light chain or sFLC testing. Nonetheless, we cannot emphasize more the importance of the standard response panel in these patients, especially with MRD as an alternative standard. The detection rate in urine was significantly lower than that in serum. This leads to efficacy overestimation, premature reduction in treatment intensity, and shortened PFS. Accurate response assessments are essential for treatment decision-making and should be standardized in all hematology centers.
AUTHOR CONTRIBUTIONS
Xialu Lan, Fujing Zhang, and Junling Zhuang wrote the first draft of the manuscript and revised the manuscript based on the authors' suggestions. Guideline panel members Chen Yang, Wei Su, Jianhua Du, Shuangjiao Liu, Miao Chen, Bing Han, and Daobin Zhou critically reviewed the manuscript and provided additional information. Junling Zhuang was the chair of the panel and led the panel meeting. All authors approved the content.
ACKNOWLEDGMENTS
This study was funded by the Capital Health Development Scientific Research Fund (Grant No. 2022-2-4013) and National High Level Hospital Clinical Research Funding (2022-PUMCH-B-048).
CONFLICT OF INTEREST STATEMENT
Professor Junling Zhuang is a member of Chronic Diseases and Translational Medicine editorial board and is not involved in the peer review and decision process of this article. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
The trial was approved by Peking Union Medical College Hospital, and informed consent form from patients was waived as it was a retrospective study.
Open Research
DATA AVAILABILITY STATEMENT
For original data, please contact the correspondence author.




