The earlier, the better? A review of neoadjuvant immunotherapy in resectable non-small-cell lung cancer
Edited by Yi Cui
Abstract
Immune checkpoint inhibitors (ICIs) have revolutionized the approach to advanced and locally advanced non-small-cell lung cancer (NSCLC). Antibodies blocking inhibitory immune checkpoints, such as programmed death 1 (PD-1) and its ligand (PD-L1), have remarkable antitumor efficacy and have been approved as a standard first- or second-line treatment in non-oncogene-addicted advanced NSCLC. The successful application of immunotherapy in advanced lung cancer has motivated researchers to further evaluate its clinical role as a neoadjuvant setting for resectable NSCLC and for improved long-term overall survival and curative rates. In this review, we discuss the efforts that incorporate ICIs into the treatment paradigm for surgically resectable lung cancer. We reviewed the early-phase results from neoadjuvant clinical trials, the landscape of the majority of ongoing phase III trials, and discuss the prospects of ICIs as a curative therapy for resectable lung cancer. We also summarized the potential biomarkers and beneficiaries involved in the current study, as well as the remaining unresolved challenges for neoadjuvant immunotherapy.
Highlights
Neoadjuvant immune monotherapy or combination therapy was tolerated, safe and associated with a high major pathological response rate, and do not result in treatment-related surgical delays.
1 INTRODUCTION
Lung cancer has the highest mortality rate worldwide.1, 2 Resectable non-small-cell lung cancer (NSCLC) is currently considered a potentially curable disease. However, only about 20% to 30% of newly diagnosed patients with lung cancer have a chance to undergo surgery with curative intent, and many of them have a high risk of recurrence (25%–70%) due to the presence of preoperative micrometastases.3 Depending on the pathological stage, the 5-year overall survival (OS) of resected non-metastatic NSCLC decreases from 73% to 24% with an increase in stage IB to IIIB.4 Distant metastasis is the leading cause of postoperative recurrence of lung cancer. Therefore, preoperative neoadjuvant therapy may be indispensable to improving long-term outcomes. A systematic review and meta-analysis indicated that neoadjuvant chemotherapy could significantly improve OS (5% at 5 years), time to distant recurrence, and recurrence-free survival (RFS) in resectable NSCLC.5 A phase III randomized trial demonstrated that adding radiotherapy to induction chemotherapy did not improve event-free survival (EFS) or OS in stage IIIA/N2 NSCLC.6 Therefore, novel neoadjuvant regimens need to be further explored. Recently, immune-checkpoint inhibitors (ICIs) have revolutionized lung cancer care in the metastatic NSCLC setting and can be considered as a therapeutic milestone.7, 8 ICIs that target cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1), and its ligand (PD-L1) have higher response rates, improved OS, and better tolerability than conventional cytotoxic chemotherapy. Currently, ICIs have already been promoted from a later line to the first-line setting for advanced patients with non-oncogene-addicted NSCLC, either alone or in combination with chemotherapy.9 To improve the long-term survival of immunotherapy in advanced NSCLC, ICIs have already been investigated to explore their efficacy in resectable NSCLC (stage IB, II, IIIA, and selected IIIB) to improve long-term OS and cure rates, including neoadjuvant treatment. Trials of neoadjuvant immunotherapy for resectable NSCLC are currently underway. In this article, we review the data from clinical trials supporting neoadjuvant immunotherapy in resectable NSCLC.
2 RATIONALE FOR NEOADJUVANT IMMUNOTHERAPY IN NSCLC
Neoadjuvant treatment aims to improve surgical outcomes for patients with resectable or potentially resectable disease, including potential tumor downstaging, increasing R0 resection rate, eradicating micrometastases, and reducing the risk of distant recurrence. At the same time, neoadjuvant treatment may preliminarily explore the therapeutic response of an individual patient, provide sufficient time to identify unsuspected metastases, and prevent unnecessary surgery. The neoadjuvant therapy phase can also be an opportunity to uncover additional comorbidities, the management of which can lead to safer radical surgery or permit efficient planning of nonoperative therapies. Generally, systemic therapy is better tolerated before surgery, and consequently, a full dose is administered to explore the therapeutic response of an individual patient.
Pathologic changes in primary tumors provide an intuitive and reliable way to assess the impact of neoadjuvant treatment on tumors and lymph nodes. Neoadjuvant immunotherapy provides a critical “window” for examining pathologic features associated with response, delineating the immunologic landscape for sensitivity and resistance to immunotherapy, and interpreting the phenomenon of pseudoprogression. Major pathological response (MPR), defined as less than 10% residual viable tumor after neoadjuvant therapy, was validated as a surrogate of survival in patients treated with neoadjuvant chemotherapy.10-12 In the era of targeted immunotherapy, the concept of MPR has been widely used, although its rationality has been questioned.13 Because of the limitation of adjuvant chemotherapy, MPR has been rare in lung cancer, with rates of 2%–7%,12, 14-17 but the incidence of MPR of neoadjuvant immunotherapy improved significantly, with a rate of 14%–83%.18, 19 The application of MPR has extended to the surrogate endpoint of neoadjuvant targeted therapy and immunotherapy clinical trials, such as the CTONG1103,20 CheckMate-159,21 Nadim,22 study, and so forth. Several trials have correlated MPR with DFS and OS in NSCLC.14-16
Theoretically, neoadjuvant immunotherapy might prime effective systemic immunity, resulting in tumor regression and improved survival. Systemic treatment before surgery might be the optimal time for immunotherapy, owing to the integrity of the primary tumor and lymphatic system. Preclinical studies of triple-negative breast cancer mouse models have shown that neoadjuvant immunotherapy improved long-term survival and enhanced antitumor immune responses compared with the same therapy administered in the adjuvant setting.23 Yonit Lavin and colleagues24, 25 showed that an immunosuppressive tumor microenvironment (TME) already exists in treatment-naïve lung adenocarcinoma lesions. The immune cell composition and phenotype in the untreated TME have changed significantly, including natural killer (NK) cells and tumor-infiltrating myeloid cells (TIM).24, 25 Lung adenocarcinoma lesions are enriched in a variety of inhibitory cells, such as PPARγhiCD64hiCD14hiPPARghiIL-6hi macrophages, CD1c+ DCs, Tregs, and exhausted T-cells, and are lack CD141+ DC, CD16+ monocytes, NK cells, and Granzyme B+ effector cells. These differences may synergistically promote an immunosuppressive microenvironment.26 The distribution of immune cell subsets did not significantly differ in the frequency and expression levels of PD-1 and PD-L1, in early and advanced stages, on macrophages and T-cells across TNM stages. Owing to the intact tumor and lymphatic/circulatory system, neoadjuvant immunotherapy can induce more activated tumor-specific T-cells to attack tumor cells and release more tumor neoantigens, which are then presented to specific effector T-cells of tumors at different sites (primary tumor, metastases, circulation). Antigen-presenting cells (APCs) recognize neoantigens and are activated. Activated APCs migrate to the lymph nodes, present neoantigens to CD4+ T-cells (MHC II) and CD8+ T-cells (MHC I), and finally activate neoantigen-specific T-cells. Activated T-cells can proliferate and infiltrate the tumor tissue to eliminate tumor cells in the primary lesion and distant micrometastases through intact blood vessels and lymphatic vessels, initiating a range of specific antitumor immune responses. Additionally, the relatively intact structure of the lymphatic and circulatory system around the tumor provides a greater chance of interaction between tumor cells and immune cells, compared with postoperative adjuvant therapy. Furthermore, the presence of a wider repertoire of tumor neoantigens can enhance immune recognition, induce a systemic and strong antitumor immune response, and produce early immune memory.26, 27 The above processes trigger a powerful systemic antitumor immune response and the generation of memory T-cells that may provide long-term protection (Figure 1).
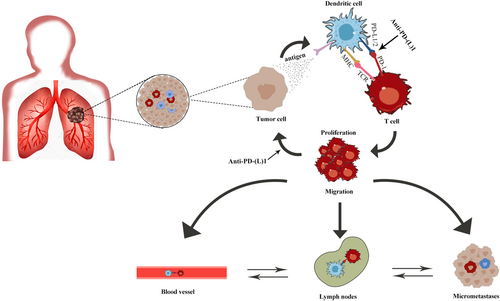
A preclinical study of breast cancer confirmed the advantages of neoadjuvant therapy with ICIs over adjuvant immunotherapy.23 TME already existed in resectable early-stage NSCLC24, 25; intact tumor and lymphatic/circulatory system provided an incubator for amplification of the immune effect. Therefore, neoadjuvant immunotherapy may be a good choice for patients with resectable early-stage NSCLC.
3 IMMUNOTHERAPY AS A NEOADJUVANT SETTING FOR RESECTABLE NSCLC
3.1 Monotherapy
MK3475-223 is a phase I study that tested neoadjuvant pembrolizumab for stage I and II NSCLC. The primary endpoints were safety, recommended phase II dose/schedule, and pathological and radiological responses. The initial result was reported in the 2018 European Society for Medical Oncology (ESMO)28 and showed that the safety profile was expected, and 66.6% of patients demonstrated a near-complete pathologic response. However, this study only recruited six patients, with only two patients who showed a response.
ChiCTR-OIC-1701372629 was a single-arm, single-center, phase Ib study that enrolled 40 patients with resectable stage IA–IIIB (8th edition of the TNM classification for lung cancer) NSCLC treated with neoadjuvant sintilimab. Postoperative pathological results indicated that 16.2% achieved a pathological complete response rate (pCR) and 40.5% achieved MPR. Radiographic assessment (RECIST 1.1) showed that the objective response rate (ORR) and the disease control rate (DCR) were 20% and 90%, respectively. Squamous cell carcinoma showed a superior response compared to adenocarcinoma (MPR 48.4% vs. 0%). A decrease in SUVmax on positron emission tomography (PET)–computed tomography (CT) (correlation coefficient = 0.86, p < 0.00001) rather than a change in the sum of lesion diameters (correlation coefficient = 0.21, p = 0.2104) was also identified as a predictor of pathological response to sintilimab in resectable NSCLC. This means that pseudoprogression should not be underestimated in neoadjuvant immunotherapy. Additionally, response heterogeneity to neoadjuvant sintilimab treatment in primary tumors and metastatic lymph nodes was observed. The indications of sintilimab in neoadjuvant therapy for surgically resectable lung cancer need to be intensively studied in the future, and key factors to overcome heterogeneous responses (pathology type, primary tumor, and lymph nodes) need to be explored. In general, sintilimab has shown good safety profiles in neoadjuvant therapy for resectable NCSLCs.
The CheckMate 159 study (NCT02259621)21 is a pilot phase II study to prospectively evaluate the safety and feasibility of neoadjuvant nivolumab in 21 patients with treatment-naive and resectable stage I–IIIA NSCLC. A total of 20 patients underwent radical surgery after neoadjuvant nivolumab treatment, and pathological analysis indicated 2 partial responses (PRs) and 18 cases of stable disease (SD); 45% of the patients achieved MPR. At follow-up, the recurrence rate within 18 months was 73% (95% confidence interval [CI], 53–100), the OS rate was 95%, and the 24-month RFS estimated by the Kaplan–Meier curve was 69%. Responses occurred in both PD-L1-positive and PD-L1-negative tumors. There was a significant correlation between pathological response and pretreatment tumor mutation burden (TMB). Although the sample size was small, this trial preliminarily confirmed the safety of neoadjuvant nivolumab for NSCLC, laying the foundation for subsequent studies.
The LCMC3 study (NCT02927301)30 is the largest phase II neoadjuvant cohort study that investigated the safety and efficacy of neoadjuvant atezolizumab in 181 patients with surgically resectable stage IB– IIIB NSCLC. After neoadjuvant atezolizumab, 43% of patients (66/155) were downstaged, while 19% of patients (29/155) were upstaged. The following operation was performed with a 92% R0 resection rate; among them, 21% and 7% of patients achieved MPR and pCR, respectively. The treatment was well tolerated, with 3% preoperative and 13% postoperative immune-related adverse events (irAEs) of grade ≥3. The 1.5-year DFS and OS rates of stage I/II and III were 79%, 91%, 77%, and 87%, respectively. Neoadjuvant atezolizumab monotherapy was confirmed to be effective and well tolerated, with no new safety signals.
The phase II randomized clinical trial, NEOSTAR,31 recruited 44 eligible patients, and 39 patients underwent curative-intent surgery. In the intention-to-treat (ITT) population of all 44 randomized patients, neoadjuvant nivolumab monotherapy induced an MPR rate of 22% and 38% after nivolumab + ipilimumab treatment. The pCR rate was 9% after nivolumab alone compared to 29% after dual therapy. Although the difference was shown numerically, no statistical significance was observed. Toxicities were manageable overall, with no new safety concerns compared with known safety profiles of nivolumab and nivolumab + ipilimumab. Grade 3–5 treatment-related adverse events (TRAEs) were reported in 13% of patients treated with nivolumab and 10% of patients treated with nivolumab and ipilimumab. The effectiveness and safety of neoadjuvant nivolumab monotherapy were similar.
The phase II multicenter PRINCEPS trial (NCT02994576)32 enrolled 30 eligible patients, and 29 patients underwent R0 resection (97%) without delay. A total of 23% of patients (7/30) developed treat-related complications a month after surgery, and grade 4–5 TRAEs were not observed. Radiological responses demonstrated 7% (2/29) PR and 93% (27/29) SD without CR and PD, respectively. Among the patients, MPR was found in 14% of patients (4/29) and pathological response ≥50% (defined as less than 50% residual viable tumor) was observed in 41% of patients (12/29). There was no correlation between MPR and radiographic changes evaluated by RECIST; there was no correlation between MPR and changes in SUVmax at 3 weeks after atelizumab treatment. The MPR was positively correlated with the expression of PD-L1 in the tumor cells before treatment.
The IFCT-1601 IONESCO33 was a phase II trial that enrolled 46 eligible patients who received neoadjuvant durvalumab. After three cycles of treatment, 89.1% of patients underwent R0 resection; among them, 8.7% achieved PR, 78.3% achieved SD, 13% achieved PD, and 18.6% achieved MPR. The 12-month OS was 89.1% [95% CI: 75.8–95.3], and the 12-month DFS was 78.2% [95% CI: 63.3–87.6]. This trial demonstrated that MPR was significantly correlated with radiographic changes and DFS, instead of OS. Unfortunately, the trial was stopped early owing to high 90-day postoperative mortality, which was possibly related to comorbidities and not direct durvalumab toxicity. A significant association was observed between MPR and DFS.
In the phase II TOP1501 trial,34 of the 35 patients enrolled, 30 received neoadjuvant pembrolizumab and 25 received surgical resection, with an 88% R0 resection rate, with 1 surgery delay. MPR was observed in 28% of the patients, including 12% pCR. The most common postoperative adverse event was atrial fibrillation, affecting 6 of the 25 patients (24%). Pembrolizumab was safe and well-tolerated in the neoadjuvant setting, and its use was not associated with excess surgical morbidity or mortality. The corresponding results of clinical trials of neoadjuvant therapy with ICIs for resectable NSCLC are detailed in Table 1.
| Identifier | Trials | Phase | Population | EGFR (sensitive)/ALK alternation exclusiveness | Sample size (N) | Intervention | Primary endpoints | Study group outcomes | Control group outcomes | ≥grade 3 irAE | Resection rate | Surgical delay rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT0293862028 | MK3475-223 | 1 | I-II | No | 28 | pembrolizumab 200 mg × 1/q3w × 2—surgery | Toxicity, MPR | 66.6% near pCR | - | NA | NA | NA |
| ChiCTR-OIC-1701372629 | / | 1b | IA-IIIB | Yes | 40 | Sintilimab 200 mg q3w × 2—surgery | Safety | MPR 40.5%pCR 16.2% | Single arm | 10% | 92.5% | 5% |
| NCT0225962121 | CheckMate159 | 2 | I-IIIA | No | 21 | nivolumab 3 mg/kg q2w × 2—surgery | Safety and feasibility | MPR 45%, pCR 10% | Single arm | 4.5% | 95% | 0 |
| NCT0292730130 | LCMC3 | 2 | IB-IIIB | No | 181 | Atezolizumab 1200 mg, q21d × 2—surgery—optional atezolizumab × 1 year | MPR | MPR 21%, pCR 7% | Single arm | 29.6% | 92.6% | 1.9% |
| NCT0315812933 | NEOSTAR | 2 | I-IIIA | No | 44 | Nivolumab 3 mg/kg q2w × 3 ± ipilimumab 1 mg/kg × 1—surgery—SOC adjuvant therapy | MPR | MPR 22%, pCR 9% | MPR 38%, pCR 29% | 13% | 89% | 22% |
| NCT0299457634 | PRINCEPS | 2 | I( ≥ 2 cm)-IIIA(non N2) | No | 30 | Atezolizumab 1200 mg, q21d × 1—surgery | Tolerance | MPR 14%pCR 16.2% | Single arm | 3.3% | 100% | 0 |
| NCT0303013135 | IFCT-1601 IONESCO | 2 | IB( > 4 cm)-IIIA (non N2) | No | 46 | Durvalumab 750 mg q2w × 3—surgery | % Of R0 resection | 89.1% R0 resectionMPR 18.6%pCR 7% | Single arm | 0 | 100% | NA |
| NCT0281892036 | TOP1501 | 2 | IB-IIIA | No | 30 | pembrolizumab 200 mg q3w × 2—surgery—SOC adjuvant therapy ± radiation + pembrolizumab 200 mg q3w × 4 | Safety and efficacy | 88% R0 resectionMPR 28%pCR 12% | Single arm | 3.3% | 83.3% | 3.3% |
- Abbreviations: MPR, major pathological response; NA, data are not available; pCR, pathological complete response; SOC, standard of care.
NEOMUN (NCT03197467) is an open-label, single-arm, prospective, single-center, ongoing phase II study. A total of 30 participants with resectable stage II/IIIA NSCLC were enrolled and administered pembrolizumab 200 mg q3w for two cycles. The primary endpoints were to assess the feasibility and safety of a neoadjuvant application of pembrolizumab and to assess the antitumor activity of pembrolizumab in terms of clinical and pathologic tumor response. The design of the NEOMUN study was consistent with traditional neoadjuvant chemotherapy, and the efficacy of neoadjuvant pembrolizumab is worth looking forward to. In Table 2, a summary of all the ongoing studies is presented.
| Identifier | Trials | Phase | Population | EGFR/ALK alternation exclusiveness | Sample size (N) | Intervention | Primary endpoint |
|---|---|---|---|---|---|---|---|
| NCT03197467 | NEOMUN | 2 | II/IIIA | No | 30 | pembrolizumab 200 mg q3w × 2—surgery—SOC adjuvant therapy ± radiation | Feasibility and safety |
| NCT03425643 | KEYNOTE-671 | 3 | II, IIIA, or IIIB (N2) | No | 786 | pembrolizumab 200 mg/placebo + platinum-based chemotherapy q3w × 4—surgery—pembrolizumab q3w × 13 | EFS, OS |
| NCT03456063 | Impower 030 | 3 | II-IIIB | Yes | 450 | Atezolizumab 1200 mg q3w+ Platinum-Based Chemotherapy/placebo × 4—surgery—atezolizumab × 16 | EFS |
| NCT03800134 | AEGEAN | 3 | II-IIIB | Yes | 800 | Durvalumab/placebo + platinum-based chemotherapy × 4—surgery—Durvalumab/placebo × 12 | pCR, EFS |
| NCT04025879 | CheckMate 77 T | 3 | IIA–IIIB (T3N2 only) | No | 452 | Nivolumab/placebo + carboplatin- or cisplatin-based doublet chemo × 4—surgery—nivolumab/placebo × 13 | EFS |
- Abbreviations: EFS, event-free survival; MPR, major pathological response; pCR, pathological complete response; PFS, progression-free survival; SOC, standard of care.
3.2 Combination therapy
Given the limited efficacy of neoadjuvant immunotherapy and the synergistic effect of chemotherapy, radiation, immunotherapy, and immunotherapy, several trials have been designed to assess the efficacy and safety of immunotherapy combined with chemotherapy and immunotherapy in the neoadjuvant treatment of early-stage NSCLC. The corresponding results of clinical trials of neoadjuvant therapy with ICIs for resectable NSCLC are detailed in Table 3.
| Identifier | Trials | Phase | Population | EGFR/ALK alternation exclusiveness | Sample size (N) | Intervention | Primary endpoint | Study group outcomes | Control group outcomes | ≥grade 3 irAE | Resection rate | Surgical delay rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT0308168924 | NADIM | 2 | IIIA | Yes | 46 | Nivolumab 360 mg + paclitaxel and carboplatin q3w × 2—surgery—nivolumab 1 year | PFS at 24 months | 89% R0 resection24 m PFS rate 77.1% MPR 83%pCR 63% | Single arm | NA | 87% | 0 |
| NCT0315812933 | NEOSTAR | 2 | I-IIIA | No | 44 | Nivolumab 3 mg/kg q2w × 3 ± ipilimumab 1 mg/kg × 1—surgery—SOC adjuvant therapy | MPR | MPR 22%, pCR 9% | MPR 38%, pCR 29% | 13% | 89% | 22% |
| NCT0336676637 | / | 2 | IB (≥4 cm)-IIIA | Yes | 13 | Nivolumab 360 mg + Pemetrexed/Gemcitabine + cisplatin q3w × 3—surgery | MPR | MPR 46%pCR 38% | Single arm | NA | NA | NA |
| NCT0257284338 | SAKK 16/14 | 2 | IIIA (N2 only) | No | 67 | Cisplatin/docetaxel × 3—durvalumab 750 mg × 2—surgery—durvalumab 1 year | EFS at 1 year | 1-year EFS 73%. MPR 62%pCR 18% | Single arm | 88% | 82% | NA |
| NCT0271603839 | / | 2 | IB-IIIA | No | 30 | Atezolizumab 1200 mg + nab-paclitaxel + carboplatin × 4—surgery | MPR | MPR 57% | Single arm | NA | 87% | 0 |
- Abbreviations: EFS, event-free survival; MPR, major pathological response; NA, data are not available; pCR, pathological complete response; PFS, progression-free survival.
3.2.1 Immunotherapy combined with chemotherapy
The NADIM study (NCT03081689)22 is the first phase II study to explore the efficacy and safety of nivolumab in combination with paclitaxel and carboplatin in neoadjuvant/adjuvant therapy in patients with resectable stage IIIA NSCLC without an EGFR-sensitive mutation or ALK alteration. After neoadjuvant combination therapy, 90% of patients had downstaging, and R0 resection was performed in 41/46 patients; MPR was 83% and pCR reached 63% after surgery; and PR was 72% and CR was 4%. Survival data showed that in the modified ITT population, the 12-month PFS was 95.7%, the 18-month PFS rate was 87.0%, the 24-month PFS rate was 77.1%, the 12-month OS rate was 97.8%, and the 18-month OS was 89.9%. 43 (93%) of 46 patients had TRAEs during neoadjuvant treatment, and 14 (30%) had TRAEs of grade 3 or worse; however, none of the adverse events were associated with surgery delays or deaths.35 In summary, the MPR and survival data of the study reached unprecedented breakthroughs and confirmed that combined immunotherapy could improve the efficacy; however, this was at the cost of a higher proportion of adverse events.
A phase II investigator-initiated trial (NCT03366766),36 which involves nivolumab plus cisplatin/pemetrexed or cisplatin/gemcitabine as induction in resectable NSCLC without an EGFR-sensitive mutation or ALK alternation, reported in the 2020 American Society of Clinical Oncology (ASCO) annual meeting, enrolled 13 patients with 6/13 (46%) MPR and 5/13 (38%) pCR, respectively. No matter of the expression of PD-L1 (positive or negative), patients adminstrated with neoadjuvant nivolumab plus platium had MPR. The radiologic response rate was 46% (PR 5, CR 1), which is consistent with the pathological response. No recurrence was observed after 10 months of follow-up. Pre-surgical grade 3 toxicity occurred in 15.4% of patients, and 1 patient died 6 weeks after surgery due to complications unrelated to the study drugs. Although this is a small study, the results indicating the effectiveness of immune combined chemotherapy are encouraging.
In a phase II study, SAKK 16/14 (NCT02572843),40 investigators should be commended for recruiting one of the largest cohorts to date of stage IIIA (N2) NSCLCs (including EGFR and ALK alterations) treated with sequential neoadjuvant chemotherapy and immunotherapy. Sixty-seven patients treated in the radiographic response rate was 58% after neoadjuvant chemotherapy and sequential immunotherapy, which improved by 15% compared to neoadjuvant chemotherapy alone, suggesting that neoadjuvant durvalumab elicited additional benefits. More than 90% of patients completed the chemotherapy and started durvalumab; 82% underwent surgical resection, and of these, 93% achieved R0 resection. In addition, 62% of the patients achieved an MPR, and 18% achieved pCR. Further analysis demonstrated that pretreatment PD-L1 expression did not affect MPR or nodal downstaging. The 1-year EFS rate was 73%. A post-hoc analysis indicated that the median EFS was significantly longer for patients achieving an MPR or pCR and nodal clearance (ypN0). A total of 88% of patients had an adverse event grade ≥ 3, suggesting that immunotherapy combined with chemotherapy increased the incidence of adverse events and was relatively severe.
A multicenter, single-arm, phase 2 trial (NCT02716038)37 enrolled 30 stage IB-IIIA NSCLC patients without identified EGFR or ALK alterations, of whom 97% (29/30) underwent surgery without delay and 87% (26/29) underwent R0 resection. The median pathological response of 29 patients who underwent R0 resection was 92.5%; 57% (17/30) and 33% (10/30) patients had MPR and pCR, respectively. No significant associations were observed between MPR or pathological complete response and pretreatment PD-L1 expression. Treatment-related grade 3–4 adverse events in this trial were consistent with published data on nab-paclitaxel and carboplatin in metastatic NSCLC.38 The overall perioperative morbidity was similar to that previously reported with neoadjuvant chemotherapy.39
Except for phase I and II clinical trials of neoadjuvant immune monotherapy or combination therapy, phase III studies have been conducted in this field. CheckMate-816 (NCT02998528)41 is the first phase III trial of nivolumab and platinum doublet chemotherapy versus chemotherapy alone, with primary endpoints of pCR and EFS. The eligible population did not enroll patients with sensitizing EGFR mutations or ALK alternations. The results indicated that the addition of nivolumab to chemotherapy increased the rate of pCR, as assessed in both resected primary lung tumors and sampled lymph nodes in the ITT population, to 24% compared with 2.2% in the chemotherapy arm. The MPR rate in the ITT population was 36.9% with the combination treatment compared to 8.9% with chemotherapy. Notably, across the neoadjuvant trials, the addition of chemotherapy to an immunotherapy agent seemed to increase the degree of pathologic regression. The incidences of grade 3–4 adverse events were 41% and 44%, and the incidences of TRAEs were 34% and 37% in the nivolumab plus chemotherapy and chemotherapy arms, respectively. Surgical outcomes for CheckMate-816 were presented at the 2021 ASCO annual meeting42 and demonstrated numbers in line with the phase II trials; definitive surgery rates were 83% with nivolumab plus chemotherapy versus 78% with chemotherapy alone, with the majority of canceled surgeries related to disease progression or other scenarios (for instance, poor lung function, or patient refusal). The results of phase III trial surgery after neoadjuvant immunotherapy did not impede the feasibility and timing of surgery, or the extent or completeness of resection versus chemotherapy alone; treatment was tolerable and did not increase surgical complications.
KEYNOTE-671(NCT03425643) is a phase III, randomized, double-blind trial of platinum doublet chemotherapy ± pembrolizumab as neoadjuvant/adjuvant therapy for patients with resectable stage II, IIIA, and resectable IIIB (T3-4N2) NSCLC, regardless of the status of EGFR and ALK. The primary endpoints were the EFS and OS. The secondary endpoints included MPR, pCR, quality of life, adverse events, perioperative complications, and treatment discontinuation due to AEs. Impower 030 (NCT03456063) was a phase III, double-blind, multicenter, randomized study evaluating the efficacy and safety of neoadjuvant treatment with atezolizumab or placebo in combination with platinum-based chemotherapy in patients with resectable stage II, IIIA, or select IIIB NSCLC, excluding non-squamous NSCLC patients with activating ALK and EGFR mutations. The primary endpoint was independent review facility (IRF)-assessed EFS. AEGEAN (NCT03800134) is a phase III, randomized, double-blind, placebo-controlled, multi-center international study assessing the activity of durvalumab and chemotherapy administered before surgery compared with placebo and chemotherapy administered before surgery in terms of a pathological complete response. Patients with EGFR mutations and ALK translocations were excluded from the trial. The primary endpoints were the pCR and EFS. The estimated enrollment was 800 participants, which is the largest in neoadjuvant immunotherapy. CheckMate 77T (NCT04025879) is also a phase III, randomized, double-blind study of neoadjuvant chemotherapy plus nivolumab versus neoadjuvant chemotherapy plus placebo, followed by surgical resection and adjuvant treatment with nivolumab or placebo for patients with resectable stage IIA (>4 cm) to IIIB (T3N2) NSCLC, regardless of the status of EGFR or ALK. To date, most phase III clinical trials are still recruiting (Table 3).
3.2.2 Immunotherapy doublet
As mentioned above, the phase II NEOSTAR study31 assessed the efficacy of neoadjuvant nivolumab (N) and nivolumab in combination with ipilimumab (NI). In 37 patients who underwent surgical resection, MPR was 29% (N vs. NI = 22% vs. 38%), and the NI group had a significantly lower percentage of viable tumor cells than the N group (20% vs. 70%, p = 0.077). Moreover, marker analysis showed that CD3 + CD103 + memory cells (81.2% vs. 54.4%, p = 0.021) and the proportion of CD8 + T-cells (56.2% vs. 38.3%, p = 0.057) significantly increased in combination immunotherapy. In terms of safety, toxicities were overall manageable, with no new safety concerns compared with known safety profiles of nivolumab and nivolumab + ipilimumab. Neoadjuvant combination therapy seems to be safe and more effective than immune monotherapy. Overall, the NEOSTAR study showed that the complexity of surgery and lung function of patients were not affected by neoadjuvant immunotherapy, and the overall resection rate was comparable to the effect of neoadjuvant chemotherapy, and there was no increase in unacceptable toxicity or perioperative morbidity and mortality. However, the fact that five patients failed to undergo surgical resection and one patient died during the perioperative period suggested that neoadjuvant immune monotherapy or combination therapy for patients with resectable NSCLC should be carefully selected after balancing the factors of treatment efficacy, surgical difficulty, and risk.
4 SAFETY OF NEOADJUVANT IMMUNOTHERAPY
Although different trials adopted different ICIs (anti-PD-L1, atezolizumab, and durvalumab; anti-PD-1, nivolumab, pembrolizumab, sintilimab, anti-CTLA-4, ipilimumab), different treatment modes (mono or combination immunotherapy), different cycles (1–4), and different populations (with or without EGFR or ALK alternation), safety analysis was necessary. The indicators for evaluating safety included the incidence of TRAEs or ICI-induced irAEs, surgical resection rate, surgical delay rate, and incidence of surgical complications. As reported, the overall incidence of grade 3 or higher irAEs ranged from 20% to 30% for patients receiving ipilimumab and from 10% to 15% for patients receiving anti-PD-1 agents, but was the highest (55%) for patients receiving anti-CTLA-4/PD-1 combination therapy.43 Although most published data on neoadjuvant combined immunotherapy in clinical trials were not available, the incidence of grade ≥ 3 irAEs presented in Table 2 seemed to be higher than 55%. Owing to unavailable data, the incidences of TRAE and surgical complications are not presented in the table. Ziran Zhao et al.44 reported the safety data of neoadjuvant ICI in 399 patients with resectable NSCLC in 10 studies, demonstrating that the average incidence of TRAEs was 32.8% (lower than > 40% with neoadjuvant chemotherapy)45 and the incidence rate of grade 3 or higher was 9%; combination immunotherapy led to an increase the incidence of TRAEs. The mean surgical resection rate was 87.5%, similar to that with neoadjuvant chemotherapy (75%–90%)45; the surgical delay rate was 1.4%, less than approximately 8% with neoadjuvant chemotherapy46, 47; and the incidence of fatal surgical complications was 1.8%, close to the minimum value of neoadjuvant chemotherapy (1%–7%).46, 47 A systematic review by Ulas et al. included 19 studies and 1066 patients and found that failure to undergo resection in monotherapy-ICI, dual therapy-ICI, chemoradiation-ICI, and chemo-ICI were 0–17%, 19%–33%, 8%, and 0–46%, respectively.48 The TRAEs grade 3 and higher rates were 0–20%, 10%–33%, 7%, and 0–67%, respectively.48 In general, the tolerance of neoadjuvant mono- or combination immunotherapy was good and had little impact on the execution of the operation.
5 PREDICTIVE BIOMARKERS OF NEOADJUVANT IMMUNOTHERAPY
The prognostic biomarkers of neoadjuvant immunotherapy currently studied mainly include PD-L1 expression, tumor mutational burden (TMB), circulating tumor DNA (ctDNA), and the tumor immune microenvironment (type and quantity of immune cells), and intertwined with each other, but the predictive value is still controversial.
In the NEOSTAR study,31 the tumor cell PD-L1 expression was overall higher in pretherapy tumor samples from patients who achieved radiographic responses (CR/PR) and MPR than in those with SD/PD and no MPR. However, there are some unmatched cases.31 MPR was positively correlated with the expression of PD-L1 in tumor cells before treatment in the PRINCEPS and LCMC3 trials. However, no correlation between PD-L1 expression and MPR was observed in CheckMate159, NCT02716038, SAKK 16/14, and NCT03366766.
Forde et al.21 found a significantly higher mutation burden in tumors with an MPR. Although no significant correlation was noted between the mutation burden and tumor PD-L1 expression, mutation burden was a primary determinant of the depth of pathological response to PD-1 blockade. The LCMC330 study also suggested that MPR was positively associated with TMB. However, the results of KEYNOTE-02149 and KEYNOTE-18950 presented in the 2019 World Conference on Lung Cancer (WCLC) revealed that TMB was not correlated with the efficacy of immunotherapy. More data are needed to explore prognostic biomarkers of neoadjuvant immunotherapy. It is worth noting that STK11 mutations were more frequent in non-MPR patients, suggesting that patients with STK11 mutations are not candidates for immunotherapy. EGFR/ALK alterations were negatively associated with MPR.51
Reuss et al.52 reported that patients who underwent pathologic review with a ≥30% reduction in viable tumor in response to nivolumab demonstrated clearance of detectable ctDNA from blood before surgery and that ctDNA might be a potential biomarker.
Bruni et al.53 summarized and analyzed 17 immune cells that might be meaningful for prognosis and found that the predictive value of immune components is related to tumor type, quantity, distribution, and activity of immune cells. The CheckMate 159 study21 also found that resected tumors with an MPR had a higher frequency of T-cell clones that were shared between intratumoral and peripheral compartments and higher clonality of the T-cell population than did tumors without an MPR. Recently, a transcriptional study of CheckMate 159 published further results, indicating that using coupled single-cell RNA sequencing and T-cell receptor sequencing, mutation-associated neoantigen (MANA)-specific CD8 T-cells were independent of tumor response (MPR or non-MPR).54
Immune profiling of resected tumors by flow cytometry and multiplex immunofluorescence (mIF) staining in the NEOSTAR study31 revealed that dual neoadjuvant ICIs could induce the proliferation of CD3+ T-cells, and the diversity of tumor-infiltrating lymphocytes (TILs) and memory TILs was also significantly increased from pre- to post-therapy. In patients who had an MPR in the LCMC3 study, T-cell activation was observed with enrichment of CD68+ and CD3+/PD-1+ T-cells, proliferation of natural killer (NK) cells, and granulocyte subpopulations, and a decrease in the monocyte subpopulation.51, 55
6 CHALLENGES OF NEOADJUVANT IMMUNOTHERAPY
First, there is no consensus on cycles of neoadjuvant immunotherapy, whether to receive adjuvant immunotherapy, the cycles of adjuvant therapy, and the optimal time of surgery. Clinicians usually empirically select the population to undergo neoadjuvant therapy based on the stage and the general condition of the patients. If the patient receives neoadjuvant chemotherapy, it usually takes two to four cycles before surgery is performed. If adjuvant treatment was performed after surgery, the same regimen was selected. The total treatment time during the perioperative period usually does not exceed four cycles. The neoadjuvant immune monotherapy or combination immunotherapy of most clinical trials mentioned above varies from two to four cycles, mainly considering not delaying the operation. Similarly, the design of clinical studies differs depending on whether patients receive adjuvant therapy after surgery. A majority of studies, including LCMC3, NEOSTAR, TOP1501, NADIM, NEOSTAR, SAKK 16/14, CheckMate 816, NEOMUN, CheckMate 77T, Impower 030, and AEGEAN, treated patients with adjuvant therapy with stand-of-care, immune monotherapy, and even combination therapy with four cycles to 1 year. Using mouse models, Liu et al.56 demonstrated that a short duration (4–5 days) between the first administration of neoadjuvant immunotherapy and resection of the primary tumor was necessary for optimal efficacy, while extending this duration (10 days) abrogated the efficacy of immunotherapy. However, efficacy was also lost if neoadjuvant immunotherapy was administered too close to surgery (2 days). Interestingly, an additional four adjuvant doses of treatment following the standard two doses of neoadjuvant immunotherapy did not significantly improve the overall tumor-free survival regardless of the combination treatment (anti-PD-1+ anti-CD137 or anti-CTLA4+ anti-PD-1). Furthermore, biochemical irAEs increased in tumor-bearing mice that received additional adjuvant immunotherapy. As far as the existing evidence is concerned, the number of cycles of neoadjuvant therapy for NSCLC and whether combination with other adjuvant therapies is required still need to be established by studies with different designs.
The second issue is the discrepancy between pathologic and radiologic diagnoses in the evaluation of the efficacy of neoadjuvant ICIs. Although most of the time, MPR and radiographic response (CR/PR) are highly consistent, some PD patients also show MPR at surgery. There were cases wherein patients achieved SD radiographically, but had achieved marked pathologic tumor regression. In the CheckMate 159 study, radiographic PR was only 10%, while the MPR was 45%. In a typical case, CT scan indicated that the targeted lesion shrunk by 35% after three cycles of neoadjuvant nivolumab, while 100% pathological regression of the large primary lung tumor was observed in the resection specimen. Another patient received two doses of nivolumab, and imaging images showed that the primary tumor was larger, whereas there was 90% tumor regression in the posttreatment specimen.21 Similar phenomena have been observed in other clinical trials, suggesting that a large number of immune cells infiltrated after immunotherapy caused the unique phenomenon of “pseudoprogression,” which also exists in early NSCLC treatment. Cascone et al.57 found a phenomenon of “nodal immune flare” (NIF), which was characterized by radiologically abnormal nodes on restaging imaging after neoadjuvant ICIs that were cancer-free and contained de novo non-caseating granulomas upon pathological evaluation. NIF was the pseudoprogression of lymph nodes, and increased immune cell infiltration was observed. The ChiCTR-OIC-17013726 study confirmed that a decrease in SUVmax was associated with pathological response to sintilimab.29 The current RECIST and MPR might not satisfy the requirements of evaluation. Instead, immune-related pathologic response criteria (irPRC)13 and the percentage change in the standard uptake value in a positron emission tomography (PET) scan58 could be used as surrogate criteria for evaluating the response to neoadjuvant immunotherapy.
In addition, as mentioned above, there is no specific biomarker to predict the therapeutic efficacy of neoadjuvant immunotherapy; therefore, the beneficial population is undefined. ChiCTR-OIC-17013726, NADIM, NCT03366766, CheckMate 816, Impower 030, and AEGEAN studies excluded sensitizing EGFR mutations and ALK translocations; however, most studies did not consider the status of EGFR and ALK. Whether they could benefit from immunotherapy, especially combined immunotherapy, needs further analysis. A single biomarker might not be adequate in predicting the efficacy of short-term immunotherapy, and multiple prognostic indicators are needed for a comprehensive evaluation.53
7 CONCLUSION
Immunotherapy has revolutionized the treatment of NSCLC in the metastatic setting and has the potential to significantly improve the outcomes of resectable early-stage NSCLC. Neoadjuvant immune monotherapy or combination therapy was tolerated, safe, and associated with a high MPR rate, especially in ICIs combined with chemotherapy. Moreover, neoadjuvant immune monotherapy or combination therapy does not result in treatment-related surgical delays. Due to the short follow-up time, the influence of neoadjuvant immunotherapy on survival needs to be confirmed by the further study results. Potential predictive biomarkers for neoadjuvant immunotherapy responses require further analysis.
AUTHOR CONTRIBUTION
Fajiu Li (First Author): Conceptualization; Literature collection; Writing-Original Draft. Ying chen: Literature collection; Writing-Original Draft. Juanjuan Wu: Literature collection and writing the outline. Chenghong Li: Resources; Supervision. Shi chen, Ziyang zhu, Wei Qin, Min Liu, Bingzhu Hu, Shuang Liu: Writing-Review & Editing. Wenzhao Zhong (Corresponding Author): Conceptualization; Funding Acquisition; Resources; Supervision; Writing-Review & Editing.
ACKNOWLEDGMENT
The funding information is not available.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
The study was a review and no ethical issues involved.
Open Research
DATA AVAILABILITY STATEMENT
All data were from the cited references.