Insulin and Metformin are Associated With Reduced Risk of Amyotrophic Lateral Sclerosis
[Correction added on 13 March 2025, after first online publication: Conflicts of Interest statement is updated.]
Abstract
Background
Type 2 diabetes (T2D), but not type 1, protected against amyotrophic lateral sclerosis (ALS). In T2D serum insulin is normal or elevated in the early stages. Type 1 diabetes, characterized by a total lack of insulin, is associated with an increased risk of ALS. The antidiabetic metformin also protects against ALS. Connexin 43 (Cx43), an astrocyte protein, operates as an open channel via which toxic substances from astrocytes reach motor neurons to cause ALS.
Methods
In the current study we analyzed FDA MedWatch data to determine whether insulin or metformin could reduce the risk of ALS. We performed in silico molecular docking studies and molecular dynamics simulation with Cx43 to determine if insulin or metformin dock within the Cx43 channel and can block it effectively, again reducing risk of ALS.
Results
In MedWatch, Insulin use is associated with a significantly reduced risk of ALS (Proportional Reporting Ratio 0.401). Metformin use is associated with a significantly reduced risk of ALS (PRR 0.567). The Human insulin heterodimer docked within center of the Cx43 channel, effectively blocking it. Molecular dynamics simulation showed that the block is highly stable and may be responsible for the protective effect of T2D on ALS. Metformin docks within the Cx43 channel, but the relatively small size of the metformin molecule may not allow it to obstruct the passage of toxic substances from astrocytes to motor neurons.
Conclusion
MedWatch data indicate that both insulin and metformin reduce risk of ALS. The results of our in silico docking study and molecular dynamics simulation corroborate our previous findings with Cx31. Insulin docks within the open hemichannel of hexameric Cx43, potentially blocking it. Molecular dynamics simulation showed that the block is stable and may be responsible for the protective effect of T2D and insulin on ALS.
Summary
-
In MedWatch, Insulin use is associated with a significantly reduced risk of amyotrophic lateral sclerosis (ALS) (Proportional Reporting Ratio 0.401). Metformin use is associated with a significantly reduced risk of ALS (PRR 0.567).
-
The Human insulin heterodimer docked within center of the Cx43 channel, effectively blocking it. Molecular dynamics simulation showed that the block is highly stable and may be responsible for the protective effect of type 2 diabetes (T2D) on ALS. Metformin docks within the connexin 43 (Cx43) channel, but the relatively small size of the metformin molecule may not allow it to obstruct the passage of toxic substances from astrocytes to motor neurons.
-
The results of our in silico docking study and molecular dynamics simulation corroborate our previous findings with Cx31. Insulin docks within the open hemichannel of hexameric Cx43, potentially blocking it. Molecular dynamics simulation showed that the block is stable and may be responsible for the protective effect of T2D and insulin on ALS. Metformin probably does not exert its protective effect by blocking the Cx43 channel.
1 Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease of motor neurons that affects up to 30,000 people in the United States each year, with 5000 new cases being diagnosed. Muscles become weaker over time, affecting physical function, and eventually leading to death. The condition has no single cause and no recognized remedy [1].
Type 2 diabetes (T2D), but not type 1, protected against ALS in a Danish population-based study [2]. In T2D serum insulin is normal or elevated in the early stages. A Swedish population study identified a significant inverse association between ALS and T2D, but not type 1 diabetes, with the strongest inverse association 6 years after diabetes onset [3]. An Italian cohort study revealed a significantly reduced ALS risk in T2D (hazard ratio 0.30) with no effect of gender, age, or ALS phenotype [4]. Zhang et al reported that genetically predicted T2D was associated with significantly lower odds of ALS both in European and East Asian populations [5]. Type 1 diabetes, characterized by a total lack of insulin, is associated with an increased risk of ALS [2, 3].
Repeat-associated non-AUG (RAN) proteins accumulate in patient brains and contribute to development of neurodegenerative diseases. The antidiabetic biguanide Metformin inhibits RAN translation through PKR pathway and mitigates disease in the C9orf72 ALS/FTD mouse model [6].
Connexin 43 (Cx43), an astrocyte protein, operates as an open channel via which toxic substances from astrocytes reach motor neurons to cause ALS. In a previous study we used in silico docking and molecular dynamics simulation with the three-dimensional structure of Cx31 as a surrogate for Cx43 to show that insulin blocks the open channel and is unlikely to be dislodged, thereby reducing risk of ALS [1].
In the current study we analyzed FDA MedWatch data to determine whether insulin or metformin could reduce the risk of ALS. The three-dimensional structure of Cx43 was deposited in the RCSB Protein Databank in March 2023. We performed in silico molecular docking studies and molecular dynamics simulation with Cx43 to determine if insulin or metformin dock within the Cx43 channel and can block it effectively, again reducing risk of ALS.
2 Methods
MedWatch is the Food and Drug Administration (FDA) Safety Information and Adverse Event Reporting Program. MedWatch was organized in 1993 to collect data regarding adverse events in healthcare. An adverse event is any undesirable experience associated with the use of a medical product. The MedWatch system collects reports of adverse reactions and quality problems, primarily due to drugs and medical devices, but also for other FDA-regulated products (e.g., dietary supplements, cosmetics, medical foods, and infant formulas) [7].
Machine-readable data from MedWatch, including adverse drug reaction reports from manufacturers, are part of a public database. We used the publicly available online tool OpenVigil [8] to query the database at https://openvigil.sourceforge.net/. For this study, drug names used were separate: insulin, metformin, and the adverse event was “amyotrophic lateral sclerosis” or “motor neuron disease.”
MedWatch results reported are the rates of the adverse event among users of a particular medication versus the rate of the adverse event among users of all other medications, as well as measures of disproportionality: observed-expected ratios like Relative Reporting Ratio, Proportional Reporting Ratio, and Reporting Odds Ratio. The Relative reporting ratio (RRR) is the ratio of how many adverse drug reactions (ADRs) under exposure were observed over the number of expected events under the assumption that ADR and drug exposure were independent. The proportional reporting ratio (PRR) is the proportion of spontaneous reports for a given drug that are linked to a specific adverse outcome, divided by the corresponding proportion for all or several other drugs. Reporting Odds Ratio (ROR) represents the odds of a certain event occurring with a medicinal product, compared to the odds of the same event occurring with all other medicinal products in the database [9]. A signal is considered when the upper limit of the 95% confidence interval (CI) of the PRR, RRR, or ROR is less than one or the lower limit of the 95% confidence interval is greater than one.
Molecular docking was done with AutoDock Vina Extended on the SAMSON platform (OneAngstrom). SAMSON is an interface for molecular design that has an open architecture and applicability for drug design [10]. AutoDock Vina Extended achieves approximately 2 orders of magnitude acceleration compared with the molecular docking software AutoDock 4 while also significantly improving the accuracy of the binding mode predictions. Further speed is achieved from parallelism by using multithreading on multicore machines. AutoDock Vina Extended automatically calculates the grid maps and clusters the results in a way transparent to the user [11].
Human insulin in vivo is a heterodimer of an A-chain and a B-chain, which are linked together by disulfide bonds. Heterodimeric human insulin was deposited in the RCSB Protein Data Bank (4EYN) January 05, 2012, released: January 05,2013 [12].
Cx43 hemi channel in nanodisc (7Z23) was deposited in the RCSB Protein Data Bank (7Z23) February 25, 2022, released: August 03, 2023 [13].
Metformin structure is from PUBCHEM Compound CID: 4091.
We used the ClusPro Server for protein-protein docking of human insulin (4EYN) to the Cx43 hexamer. ClusPro (https://cluspro.org) is a widely used tool for protein–protein docking. The server provides a simple home page for basic use, requiring only two files in Protein Data Bank (PDB) format [14]. The quality of automated docking by ClusPro is very close to that of the best human predictor groups [15].
We used GROMACS 2021.3 to perform molecular dynamics simulation of human insulin (4EYN) docked to the Cx43 hexamer (7Z23). GROMACS is a molecular dynamics package mainly designed for simulations of proteins, lipids, and nucleic acids. The all-atom OPLS-AA/L force field and SPC/E water model were used for simulations. Energy minimization was performed using the steepest descent method. System Equilibration was done in two phases. The first phase was conducted under an NVT ensemble (constant Number of particles, Volume, and system Temperature). The second phase was conducted under an NPT ensemble, wherein the Number of particles, Pressure, and Temperature are all constant. This ensemble is also called the “isothermal-isobaric” ensemble, and most closely resembles experimental conditions.
3 Results
3.1 Insulin and ALS
Insulin use is associated with a significantly reduced risk of ALS, as indicated by a Proportional Reporting Ratio (PRR) of 0.401. MedWatch data encompassing 11,737,133 subjects were analyzed to evaluate this relationship. Within this cohort, five individuals (four men, one woman) were identified with ALS, with a mean age of 59.0 ± 7.8 years. The incidence rate (DE/D) was calculated to be 0.004%. A Chi Squared test with Yates' correction yielded a value of 3.871, indicating statistical significance (values greater than 3.84 are considered significant). Disproportionality measures were as follows: Relative Reporting Ratio (RRR), 0.404 (95% CI: 0.168; 0.972); Proportional Reporting Ratio (PRR), 0.401 (95% CI: 0.167; 0.965); Reporting Odds Ratio (ROR), 0.401 (95% CI: 0.167; 0.965) (Table 1).
| Items | Insulin | All other drugs | ∑ |
|---|---|---|---|
| ALS | 5 (DE) | 1082 | 1087 |
| All other adverse events | 133712 | 11602334 | 11736046 |
| ∑ | 133717 (D) | 11603416 | 11737133 |
- Note: Insulin use is associated with a significantly reduced risk of amyotrophic lateral sclerosis PRR (0.401).
3.2 Metformin and ALS
Similarly, metformin use is associated with a significantly reduced risk of ALS, with a PRR of 0.567. In the same MedWatch data set, 15 individuals (14 men, 1 woman) with ALS were identified, with a mean age of 68 ± 21 years. The incidence rate (DE/D) for metformin users was 0.005%. The Chi Squared test with Yates' correction produced a value of 3.831, also indicating statistical significance. Disproportionality measures were as follows: Relative Reporting Ratio (RRR), 0.572 (95% CI: 0.331; 0.988); Proportional Reporting Ratio (PRR),0.567 (95% CI: 0.328; 0.979); Reporting Odds Ratio (ROR),0.567 (95% CI: 0.328; 0.979) (Table 2).
| Metformin | All other drugs | ∑ | |
|---|---|---|---|
| ALS | 13 (DE) | 1074 | 1087 |
| All other adverse events | 245468 | 11490578 | 11736046 |
| ∑ | 245481 (D) | 11491652 | 11737133 |
- Note: Metformin use is associated with a significantly reduced risk of amyotrophic lateral sclerosis (PRR 0.567).
3.3 Motor Neuron Disease Analysis
When the query was repeated with the term “amyotrophic lateral sclerosis” replaced by “motor neuron disease,” the results similarly indicated a reduced risk associated with both insulin and metformin use. Specifically: insulin, PRR of 0.331; Metformin, PRR of 0.723.
3.4 Docking Studies and Molecular Dynamics
3.4.1 Metformin Docking to Cx43
Metformin docking to Cx43 was validated using AutoDock Vina Extended, with Table 3 presenting the docking parameters. Lower RMSD values indicate higher docking accuracy. Only one docking position within the Cx43 channel (mode 1) was highly valid, with an RMSD of 0. Additional docking positions were less valid. Figure 1A depicts metformin docked to Cx43, and Figure 1B provides a closeup view showing metformin within the Cx43 channel. Figure 2 shows the binding affinity for seven docking sites, with the highest affinity and validity within the Cx43 channel.
| Ligand's mode | Affinity (kcal/mol) | Ki (umol) | Lower bound of the RMSD from this ligand's best mode (A) | Upper bound of the RMSD from this ligand's best mode (A) |
|---|---|---|---|---|
| 1 | −0.86405 | 232621 | 0 | 0 |
| 2 | −0.84751 | 239204 | 21.3587 | 22.2254 |
| 3 | −0.84059 | 242014 | 2.24824 | 4.12859 |
| 4 | −0.83202 | 245540 | 44.4595 | 45.3646 |
| 5 | −0.82685 | 247693 | 1.85216 | 3.43137 |
| 6 | −0.80206 | 258276 | 46.8599 | 48.6123 |
| 7 | −0.79966 | 259326 | 2.02379 | 4.72418 |
| 8 | −0.7968 | 260582 | 40.3854 | 42.4403 |
| 9 | −0.78197 | 267187 | 36.5951 | 37.5994 |
| 10 | −0.77487 | 270408 | 24.5007 | 25.605 |
- Note: Lower values of root-mean-square deviations of atomic positions (RMSD) indicate that docking is validated with higher accuracy. RMSD values of 3 or more indicate no docking has occurred. One docking position, mode 1, with RMSD = 0 is highly valid.

3.4.2 Insulin Docking to Cx43
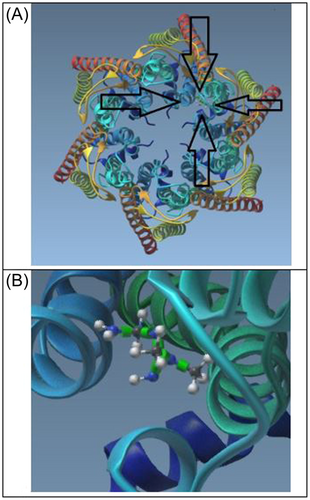
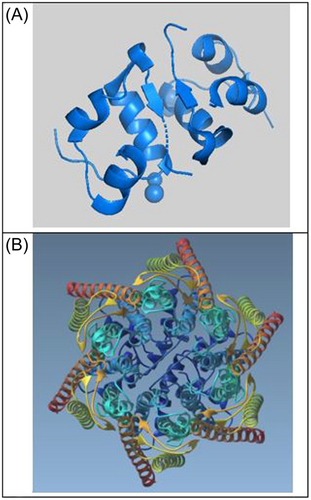
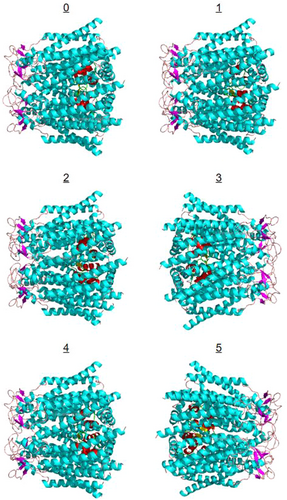
Figure 3A shows the Human insulin heterodimer (4EYN), and Figure 3B illustrates the insulin heterodimer docked within the center of the Cx43 channel. ClusPro results in Figure 4 show the first six configurations of Human Cx43 in its hexameric form with insulin docked within the hemichannel. Configuration 0, the highest ranked, is enlarged and rotated in Figure 3B, demonstrating the insulin heterodimer consistently blocking the Cx43 hemichannel in all configurations. Table 4 presents cluster scores and energies for these configurations, with cluster rank determined by the position with the most neighbors within 9 angstroms.
| Cluster | Members | Representative | Weighted Score |
|---|---|---|---|
| 0 | 176 | Center | −1692.2 |
| 0 | 176 | Lowest Energy | −2255.5 |
| 1 | 152 | Center | −1719.6 |
| 1 | 152 | Lowest Energy | −2309 |
| 2 | 124 | Center | −1720.7 |
| 2 | 124 | Lowest Energy | −2278.6 |
| 3 | 93 | Center | −1814.8 |
| 3 | 93 | Lowest Energy | −2294.8 |
| 4 | 91 | Center | −1695.2 |
| 4 | 91 | Lowest Energy | −2284.1 |
| 5 | 73 | Center | −1758.9 |
| 5 | 73 | Lowest Energy | −2240.8 |
- Note: The ligand position with the most neighbors within nine angstroms becomes a cluster center, and its neighbors the members of the cluster. These are then removed from the set and a second cluster center is located, then a third, up to cluster 5. Thus, the cluster rank is determined.
3.4.3 Molecular Dynamics Simulation
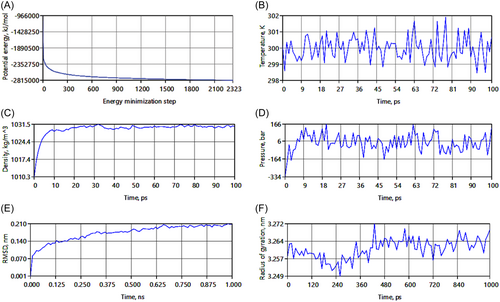
Figure 5 summarizes the molecular dynamics simulation results. Figure 5A shows a smooth and steady convergence of the system's potential energy (Epot). Figure 5B demonstrates that the temperature stabilizes around 300 K. Figures 5C and D indicate that the application of pressure stabilizes the system density at 1030 kg/m³, which is close to the expected value. Figure 5E shows the time series of RMSD levels (~0.1 nm), indicating structural stability. Finally, Figure 5F reveals that the radius of gyration (Rg) remains invariant, suggesting stability over 1 ns. These results suggest that once insulin blocks the Cx43 hemichannel, the blockage remains stable.
4 Discussion
Connexin channels are proteins that form gap junctions and hemichannels in astrocytes and play a crucial role in the maintenance of the normal functions of the Central Nervous System (CNS). Alterations of astrocytic connexin expression and function in neurodegenerative diseases have been shown to affect disease progression by changing neuronal function and survival. In ALS, Cx43 gap junctions and hemichannels mediate astrocyte intercellular communication in the CNS under normal conditions and may contribute to astrocyte-mediated neurotoxicity. Targeting connexins can be a plausible therapeutic strategy to manage neurodegenerative diseases, including ALS [16, 17].
Cx43, an astrocyte protein, operates as an open pore via which toxic substances from astrocytes reach motor neurons to cause ALS. We previously performed molecular docking of insulin with monomeric Cx31, monomeric Cx43, and hexameric Cx31 to assess whether insulin might affect the pore. Hexameric Cx31 and hexameric Cx43 are transmembrane hemichannels composed of 6 subunits; they bind together to form gap junction intercellular channels. We used the program AutoDock Vina Extended for the molecular docking study. Cx31 shares amino acid and structural similarity to Cx43, and insulin docks to the same position at the N-terminal domain of monomeric Cx31 and monomeric Cx43. We found that insulin docks within the open hemichannel of hexameric Cx31, potentially blocking it. Molecular dynamics simulation showed that the block is highly stable and may be responsible for the protective effect of T2D on ALS [1]. MedWatch data presented above in Table 1 confirm the protective effect of insulin.
The full Cx43 hexameric structure was deposited in the RCSB Protein Data Bank and released 8 March 2023. The results of our in silico docking study and molecular dynamics simulation confirm our previously reported findings [1]. We found that insulin docks within the open hemichannel of hexameric Cx43, potentially blocking it. Molecular dynamics simulation showed that the block is highly stable and may be responsible for the protective effect of T2D on ALS.
C-9 ALS is a subtype of ALS caused by a repeat expansion mutation in a gene on chromosome 9, open reading frame 72 (C9orf72). The mutation occurs when six letters of DNA – GGGGCC – are repeated hundreds of times. Besides ALS, mutations in C9orf72 can cause frontotemporal dementia. Some patients with the C9orf72 mutation develop ALS, others develop frontotemporal dementia, and some develop both. MedWatch data (Table 2) corroborate the protective effect of metformin identified in the C9orf72 ALS/FTD mouse ALS model [6].
Metformin inhibits protein kinase R, reduces RAN proteins, and improves disease in C9-ALS/FTD mice. Metformin might be therapeutic for this genetic form of ALS and frontotemporal dementia because the C9orf72 mutation makes RAN proteins [6]. But metformin treatment was not effective in mice with a different form of ALS that does not produce RAN proteins [18].
Our finding that metformin docks within the Cx43 channel suggests that in some cases metformin may interfere with the passage of toxins through this channel from glial cells to motor neurons. However, because of its relatively small size compared with insulin, metformin could obstruct the Cx43 channel much less completely than insulin. Metformin does penetrate the blood brain barrier and seems to exert neuroprotective, anti-inflammatory, and antioxidant effects, including prevention of dementia and neurodegenerative diseases in humans [19, 20].
Molecular docking studies are a powerful in silico approach for discovering novel therapies for unmet medical needs by predicting drug–target interactions. But molecular docking studies are not a substitute for in vitro studies. In vitro studies are conducted in a controlled environment, such as a test tube or petri dish, and can provide more accurate results than molecular docking studies. In vitro studies can provide information about the drug's efficacy, toxicity, and pharmacokinetics, which are essential for drug development. But molecular docking studies can provide a preliminary assessment of the drug's potential efficacy and binding affinity with the target protein. Molecular docking studies are a valuable tool for drug discovery used in conjunction with in vitro studies to validate the results and ensure the safety and efficacy of the drug [21].
Molecular dynamics simulation is a computational technique that can provide mechanistic understanding of molecular systems and has become a prominent tool in pharmaceutical research. It can provide insight into the behavior of molecules at an atomic level that is difficult to characterize experimentally. However, molecular dynamics simulations are not a replacement for in vitro studies. In vitro studies are conducted in a controlled laboratory environment and can provide more accurate results than simulations. Molecular dynamics simulations can provide valuable insights into molecular systems but should be used in conjunction with in vitro studies to ensure the accuracy of the results [22].
Our study has some weaknesses. A MedWatch report of an adverse event does not establish causation. For any given report, there is no certainty that the drug in question is related to the reaction. The adverse event may have been due to the underlying disease being treated, another drug being taken concurrently, or something else. The MedWatch data are imperfect, with under- and over-reporting, missing denominator (i.e., number of doses for a drug), wrong, duplicate and/or missing data in the database [23]. Consequently, the total number of adverse event reports for all drugs and/or the drug in question from OpenVigil can vary slightly from drug to drug and for different adverse events related to the same drug. The imperfect MedWatch data have presented a problem that all analytical software programs, such as OpenVigil, have been forced to confront [24].
In the MedWatch query we could not determine whether cases were all type 2 diabetes patients, or whether they included type 1 diabetes, type 2 diabetes, or other non-diabetes subjects who used insulin and metformin.
A large proportion—about 40%—of people with type 2 diabetes who were prescribed second-line medications after taking metformin to manage their condition stopped the drugs within 1 year, according to a retrospective cohort study that involved more than 82 000 insured participants between 2013 and 2017 [25, 26]. Nearly two-thirds modified their treatment in some way, including switching or intensifying their medications. About half of the people who stopped taking their medications had been prescribed glucagon-like peptide-1 (GLP-1) receptor agonists. These modifications could skew or distort MedWatch results reported here.
It would be worthwhile to use the MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities shown in Table 3 [27].
Cx43 (7Z23) structure is presented in the closed position. Therefore, we cannot be certain of the insulin blocking effect when Cx43 is in the open position.
5 Conclusion
MedWatch data confirms the protective effect of insulin and metformin against ALS. The results of our in silico docking study and molecular dynamics simulation corroborate our previous findings with Cx31. We now report that insulin docks within the open hemichannel of hexameric Cx43, potentially blocking it. Molecular dynamics simulation showed that the block is highly stable and may be responsible for the protective effect of T2D and insulin on ALS. Metformin probably does not exert its protective effect by blocking the Cx43 channel [28].
Author Contributions
Steven Lehrer and Peter H. Rheinstein contributed equally to the conception, writing, and data analysis of this study.
Acknowledgments
The authors have nothing to report.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
Steven Lehrer serves in a scientific advisory capacity with Fermata Pharma Inc. Peter H. Rheinstein is President of Severn Health Solutions. The scientific content was developed without influence from Fermata Pharma Inc. or Severn Health Solutions.
Open Research
Data Availability Statement
Data sources described in the article are publicly available. This article was previously available as a preprint on http://researchsquare.com/article/rs-3860653/v1. The current version has been peer-reviewed and accepted.




