Accurate and Safe Directional Coronary Atherectomy Using the X-Ray Detector's Calculated Optimal Angular View
ABSTRACT
Background
Directional coronary atherectomy (DCA) is effective for bifurcation lesions but carries a risk of coronary perforation, due to geographical and recognition errors during the procedure. Geographical errors involve incorrect targeting of plaque-free areas, whereas recognition errors stem from the misinterpretation of visual information during catheter alignment. To minimize these risks and improve procedural safety and efficacy, it is crucial to align the DCA catheter accurately with the target plaque by utilizing the morphological characteristics of the housing.
Aims
This study aimed to determine whether visual recognition of DCA housing from the front or side, as opposed to an oblique angle, reduces cutting errors.
Methods
Fifteen physicians and five medical staff members performed a bench test in which a DCA catheter was rotated 0° (frontal view), 45° clockwise (oblique view), and 90° clockwise (lateral view) under fluoroscopy. The error angles at each position were compared (p < 0.05).
Results
Error angles were 4.4 ± 3.4° (frontal view), 10.1 ± 4.6° (lateral view), and 13.6 ± 7.5° (oblique view). The frontal view showed fewer errors than the lateral and oblique views (p = 0.0016 and p < 0.0001, respectively). No significant differences were observed between the physicians and medical staff.
Conclusion
Recognizing DCA housing from the frontal or lateral view calculated from intravascular ultrasound (IVUS) significantly reduces recognition errors in plaque cutting, enables safer and more effective procedures, even for less experienced operators, and has the potential to improve the safety and outcomes of DCA procedures.
Abbreviations
-
- DCA
-
- directional coronary atherectomy
-
- IVUS
-
- intravascular ultrasound
-
- PCI
-
- percutaneous coronary intervention
1 Introduction
Directional coronary atherectomy (DCA) was introduced in 1990 as a novel percutaneous revascularization technique. Initially, it was used for large coronary vessels that were free of tortuosity and calcification. The overall outcomes demonstrated a 96% procedural success rate, an 82% clinical success rate, and an 11% incidence of major complications (death, emergency bypass surgery, acute myocardial infarction, abrupt vessel closure) [1]. Coronary perforation is one of the most serious complications of DCA. Its incidence rate is approximately 0.4%−1.4% [1, 2], which is not particularly high compared to that of other procedures; however, it can rapidly lead to cardiac tamponade and can sometimes be fatal. One cause of coronary perforation is geographical errors, which include longitudinal geographical errors caused by the incorrect cutting of plaque-free areas in the long-axis direction and circumferential geographical errors caused by the incorrect cutting of plaque-free areas along the short axis. Typically, the housing direction is evaluated from two different perspectives to prevent this error. However, recognition errors may occur in the process of applying visual information about the distribution of the target plaque in the cutting direction, owing to the tortuosity of the target vessel or the branching position of the side branches. To avoid these errors, it is imperative to compare and synchronize the findings of intravascular ultrasound (IVUS) and angiography. The more accurately the IVUS images are synchronized with angiographic findings, the more clearly the plaque distribution can be visualized along both the longitudinal and circumferential axes. Several established methods are commonly employed to match the target plaque distribution with fluoroscopic information in the DCA procedure.
Several techniques have been developed to minimize geographic errors in DCA procedure, including the side branch method, guide wire bias method, coincident point method (using the alignment of a guide wire and IVUS transducer), and the guide wire tip detection method [3, 4]. Geographic misses often occur when fluoroscopic visual information is not accurately applied to the spatial distribution of the target plaque. Among the four methods mentioned above, the guidewire tip detection method can determine the direction more accurately, irrespective of the intersection of the guidewire and the IVUS catheter or the presence or absence of side branches. The operator rotates the guidewire clockwise to align the tip and shaft in a straight line with the fluoroscopic observation direction and ensures that the guidewire tip direction in the IVUS short-axis image matches this observation direction. Therefore, it is possible to comprehend the distribution of any given plaque by matching the direction in which the guidewire tip and shaft appear to be in a straight line with the IVUS information (Figure 5).
In addition to these methods, a novel approach that emphasizes a shift in perspective has been proposed to minimize recognition errors. It is common to assess the housing direction from two different perspectives: the right anterior oblique caudal and left anterior oblique caudal, when cutting a lesion in the proximal part of the left anterior descending artery [5]. If the target plaque cannot be observed from the front or side in either of these directions, even if the tip detection method is used, the rotation of the housing must be fine-tuned, which may not only lack accuracy but also compromise safety. Nevertheless, unintended rotational misalignment of the housing sometimes occurs owing to a rotational torque surplus in the DCA catheter or slippage between the DCA balloon and plaque. The housing of the DCA catheter is boat-shaped; therefore, it appears rectangular from the front and concave from the side aspect. The other oblique directions are represented as fluoroscopic shilhouettes with various combinations of parallelograms and concave surfaces. If the target plaque is located in an area except the 12, 3, 6, and 9 o'clock directions, which are synchronized with IVUS, it is unclear whether the housing will accurately capture the plaque. Therefore, if the X-ray detector could be moved to an angle that always provides only a frontal or lateral view of the housing for the target plaque, it would be possible to cut the target plaque in a more accurate direction. Moreover, viewing the housing from the front or side aspect may also improve accuracy and safety by making it easier to recognize any misalignment due to malrotation of the catheter immediately before balloon expansion. This study aimed to evaluate that visual recognition of housing viewed from the front or side angle results in fewer errors than that from an oblique angle during DCA.
2 Methods
2.1 Apparatus
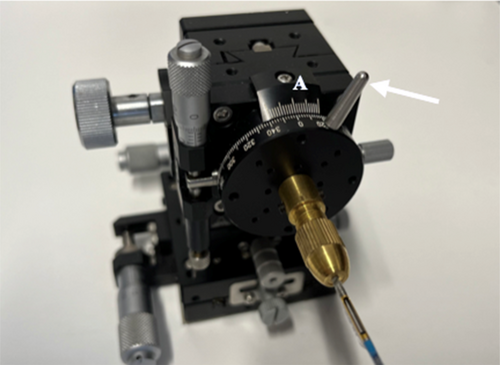
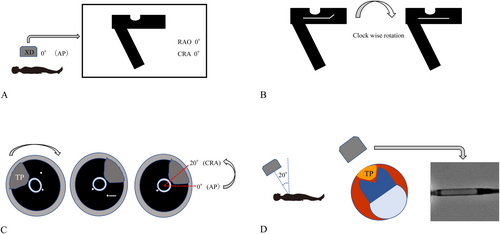
This study utilized a custom-made apparatus assembled from multiple brass components (B23-60C, B54-40U2N, A47-106, B05-1NHM, B43-38N, Suruga Seiki, Shizuoka, Japan), which can be fixed and adjusted at any desired position, as shown in Figure 1. The device measures 100 mm in height, 60 mm in width, and 60 mm in depth. Its front features a 360° protractor with 2° increments, which can be rotated by manipulating a silver-colored knob (Figure 1: white arrow). Aligning the 0° marks on scale A with the center indicates that the topmost position corresponds to 0°. A gold-colored protrusion is positioned centrally on the 360° protractor and is designed to secure the catheter in place via a screw mechanism. The housing of the DCA catheter is mounted on this protrusion such that it faces 0° or directly upward.
2.2 Procedure
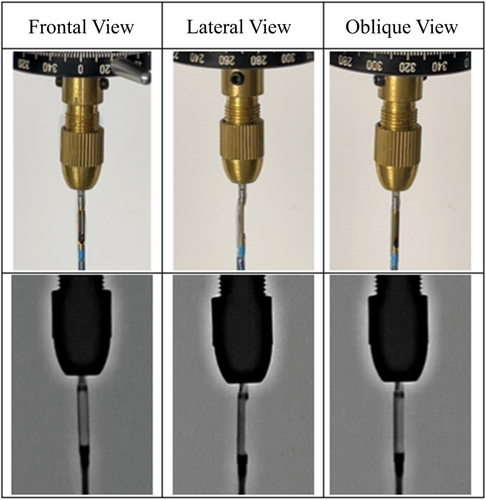
The apparatus was placed on an operating table, and a spirit level was used to ensure horizontal alignment. This experiment was conducted with 15 physicians from our institution, including six cardiologists and nine from non-cardiology specialties, as well as five medical staff members from our catheterization laboratory. The 360° rotating plate was rotated clockwise using a silver-colored knob. Using only visual information from the housing's shape, as observed under fluoroscopy, the housing was aligned to face directly forward (0°), at a 45° oblique angle, and at a 90° lateral angle, as shown in Figure 2. The angular deviation from the measured position was recorded for each alignment. Before starting the experiment, all participants received brief instructions on its purpose, the operation of the apparatus, and the appearance of the housing under fluoroscopy.
2.3 Statistical Analysis
Statistical significance was set at p < 0.05. Differences between groups were assessed using the Student's t-test for continuous variables, the Mann–Whitney U test for ordinal variables, and the chi-square test for categorical variables. All statistical analyses were performed using JMP software (JMP version 17.2.0; SAS Institute, Cary, NC, USA).
3 Results
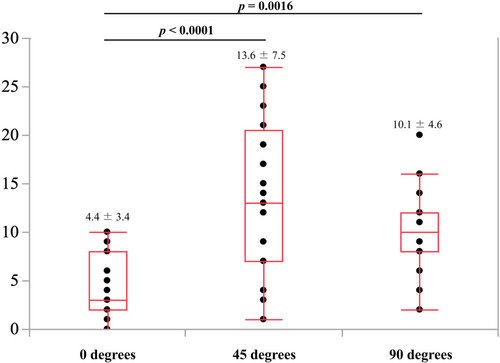
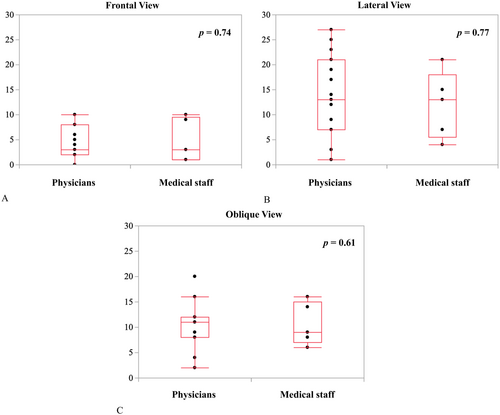
The recognition errors were 4.4 ± 3.4°, 10.1 ± 4.6°, and 13.6 ± 7.5° in the frontal, lateral, and oblique views, respectively. The recognition error in the frontal view was significantly lower than those in the lateral and oblique views (p = 0.0016 and p < 0.0001, respectively) (Figure 3). Furthermore, the error for physicians versus medical staff members were 4.2 ± 3.1° versus 4.8 ± 4.4° (p = 0.74) in the frontal view, 9.9 ± 4.9° versus 10.6 ± 4.2° (p = 0.77) in the lateral view, and 14.1 ± 7.9° versus 12.0 ± 6.7° (p = 0.61) in the oblique views (Figure 4A−C). No significant differences were observed between the two groups in any of the views.
4 Discussion
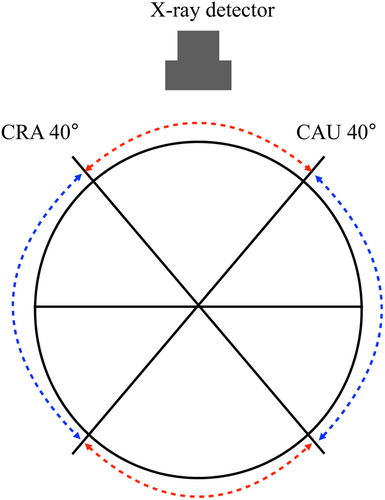
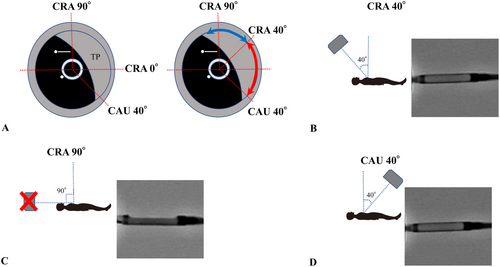
Bifurcation lesions account for 15%−20% of percutaneous coronary intervention (PCI) cases [6], and achieving initial success and maintaining long-term patency are challenging [7]. The greater the residual plaque burden, the higher the rate of restenosis [8]. Therefore, plaque debulking is considered highly beneficial. However, debulking with rotational atherectomy or orbital atherectomy system carries a higher risk of no-reflow or slow-flow due to excessive tissue removal. In contrast, DCA minimizes these risks and improves long-term outcomes [9], allowing for the avoidance of complex bifurcation stenting procedures. From a clinical research perspective, DCA is the only technique that enables the acquisition of plaque samples for histopathological analysis. Despite these advantages, DCA remains underutilized due to its complexity, which often deters beginners. If the DCA procedure could be simplified and performed more safely and efficiently, both initial success rates and long-term outcomes could be improved. Consequently, we conducted this experiment to increase the accessibility of DCA. Notably, the lack of significant differences between DCA-experienced operators and DCA-unfamiliar medical staff suggests that once the housing's shape is learned, visual recognition becomes relatively intuitive. The excellent visibility from the frontal view is likely due to the symmetrical structure of the housing and the alignment of its support body with the guidewire. For precise plaque targeting, it is essential to match the fluoroscopy direction observed by the operator with the IVUS information. Suzuki et al. described a method in which, during DCA of a lesion in the proximal left anterior descending coronary artery, the guidewire is rotated 90° clockwise from the position where the tip of the guidewire is at the top. The IVUS image is then rotated so that the guidewire tip appears at the 3 o'clock position on the IVUS short-axis view. This aligns the IVUS and the fluoroscopic images and is known as the guidewire tip detection method [3]. After identifying the location of the target plaque using this method, the X-ray detector can be positioned to enable visualization of the housing of DCA either frontally or laterally, minimizing reliance on oblique view and reducing angular recognition errors (Figure 5). Furthermore, the angular range of plaque excision in a single session can be calculated based on the DCA catheter geometry. Assuming a rectangular housing opening is 1.38 mm and a vessel radius of r, the angle θ can be calculated using the cosine theorem: cos θ = 1−0.952/r^2. This yields excision angles of 55°, 46°, 40°, and 36° for vessel diameters of 3.0, 3.5, 4.0, and 4.5 mm, respectively. This theory estimates the minimum angle at which the DCA catheter must be rotated for the next cut, thereby allowing continuous plaque cutting with minimal rotation. Although the X-ray detector's range of motion is limited to approximately 40° in the cranial-caudal direction, this still allows for direct frontal visualization at 160° (mirror of 80°), shown by the red dotted arc in Figure 6. However, the remaining 200° (mirror of 100°), shown in blue, cannot be visualized frontally. In such cases, lateral views should be used, as they provide the second-lowest recognition error. The lateral view is achieved by rotating the catheter such that the housing of DCA is visualized fully from the side, with the X-ray detector offset 90° from the target plaque. With this approach, by allowing intentional cutting in any direction across the full 360° is feasible using frontal and lateral views alone (Figure 7). A biplane X-ray system further enhances this approach by allowing real-time confirmation of balloon expansion from multiple angles, reducing the need to reposition the X-ray detector and shortening balloon occlusion time. Our method identifies optimal frontal and lateral fluoroscopic angles for visualizing the housing, based on IVUS data. Since this process requires no disruption of workflow or specialized training, it can be implemented using existing equipment with only minimal additional training. The only potential requirement is some knowledge about this method and slight training to align at the IVUS information with the fluoroscopy direction at the default angle, which is much easier compared to the training required to master the existing other cutting method. This method is expected to reduce recognition errors significantly. Even less experienced operators may be able to perform DCA more safely and effectively with an adequate understanding of our technique. Nevertheless, anatomical complexities such as tortuosity or heavy calcification remain potential limitations for the broader application of DCA, including with our proposed method. Unlike rotational or orbital atherectomy systems, DCA is generally less effective for heavily calcified lesions, which are typically considered unsuitable for this technique. In addition, the structural rigidity of the DCA catheter can make it difficult to navigate tortuous coronary segments, especially in the proximal left coronary artery. As a result, DCA and similar methods are often limited to more proximal lesions. However, if the catheter can be successfully advanced to the target site, our technique—based on IVUS-guided alignment and optimal fluoroscopic visualization—could theoretically be applied to any coronary lesion. Further clinical evaluation in anatomically challenging scenarios is warranted to fully assess its feasibility and benefits in such cases. The method is also expected to be highly effective, even for experienced operators, as a technique to precisely remove residual plaque or intimal flap in a short period after initial cutting.
5 Limitations
This study has several limitations that should be considered. First, because this was a bench test, it is not yet determined whether this method is effective (intraoperatively) within the coronary arteries during the DCA procedure. Frictional resistance within the guiding catheter and the atherosclerotic coronary arteries may hinder the precise rotation of the DCA catheter, making it challenging to maintain its intended alignment with the target plaque. Our method utilizes an existing DCA catheter and is not a technique that involves the first use of a new device in the human body. The DCA catheter and the excision method adhere to conventional procedural principles, requiring no additional devices and only minimal technical adaptation. The key aspect of our method does not introduce a new device or technique but instead improves safety by shifting the perspective on plaque excision. Therefore, major concerns regarding clinical application are anticipated, but further clinical validation with is required. Second, another issue is the substantial difference in operator experience with DCA catheter. Although there are 15 physicians from our institute, only two are experienced in using DCA catheters. Additionally, three out of five medical staff members who were familiar with supporting DCA procedures had never handled a DCA catheter. However, this consideration is closely related to shape recognition, which is a part of human recognition skills. Once the housing structure is understood, the level of familiarity with the device becomes less relevant. The final limitation is that DCA is predominantly performed in Japan and has not yet gained widespread adoption globally. As a result, the amount of evidence and optimization of this procedure is relatively limited compared to other procedures. However, if our method gains wider recognition, it could contribute to the global adoption of DCA.
6 Conclusions
The technique of dynamically adjusting the X-ray detector to maintain a frontal view of the DCA catheter housing relative to the target plaque may enhance the accuracy and safety of DCA procedures compared to conventional methods. This approach could potentially facilitate the broader application of DCA in clinical practice, enabling less experienced operators to perform the procedure with greater confidence and improved outcomes.
Clinical Perspectives
What is Known?
Several methods exist for identifying the optimal cutting DCA procedure; however, these techniques often lack precision.
What is New?
In this preliminary study, we developed a procedure that entails a paradigm shift from traditional methods of fixing the fluoroscopy angle to dynamically adjusting the fluoroscope to the optimal angle calculated based on IVUS data, which represents a significant advancement.
What is Next?
The results of this study must be applied in clinical practice, and future studies should validate the safety of this procedure.
Acknowledgments
The authors are deeply indebted to Seiichiro Murano for his critical mechanical support, without which this experiment would not have been possible. We would also like to express our sincere gratitude to the physicians and staff of the Catheterization Laboratory at Sendai Open Hospital for their participation in and support of this experiment.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the supporting information of this article.




