Stent Fracture of Biolimus-Eluting Stent (Biofreedom) Complicated With Micro-Coronary Artery Aneurysm
ABSTRACT
Coronary stent fractures (SFs) are an emerging complication of coronary stents. There are numerous risk factors for SF, which include vessel angulation, use of sirolimus-eluting stent (SES), long stents, multiple stent layers, stent overlap, inflammation, hypersensitivity, stenting in the right coronary artery, heavy calcification, ostial bifurcational lesions, and stent overexpansion. There have been reports of SF in BES (Nobori) in past studies, but no reports on BES (Biofreedom) have been documented yet. SF increases the risk of in-stent restenosis, stent thrombosis (ST), target lesion revascularization and major adverse cardiac events. A coronary artery aneurysm (CAA) is a rare complication of SF. Although the risk of CAA rupture with SF is high, the treatment and management of CAA with SF remain unclear. Here, we report the case of a 72-year-old man who had SF of a BES (Biofreedom) complicated with a micro-CAA and review some of the literature.
1 Introduction
The incidence of drug-eluting stent (DES) fracture in current registries was reported to range from 1.7% to 2.6% [1]. Regarding biolimus-eluting stent (BES), the SF of Nobori had been reported, however, there have been no previous reports of stent fracture (SF) in Biofreedom [2].
2 Case Report
A 72-year-old man was transferred to our hospital due to typical chest pain with elevation of Troponin T and no ST-segment elevation on electrocardiography. Comorbidities included diabetes mellitus, chronic kidney disease, hyperlipidemia, and smoking history.
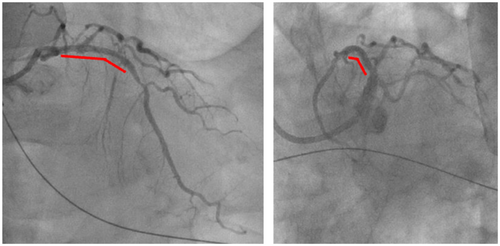
He had undergone primary percutaneous coronary intervention (PCI) for the mid-portion of left anterior descending artery (LAD) to treat acute anterior myocardial infarction using a BES (Biofreedom 3.0 × 36 mm) 14 months ago (Figure 1). Thereafter, the patient continued dual antiplatelet therapy for 3 months, followed by single antiplatelet therapy, with good adherence and no missed doses.
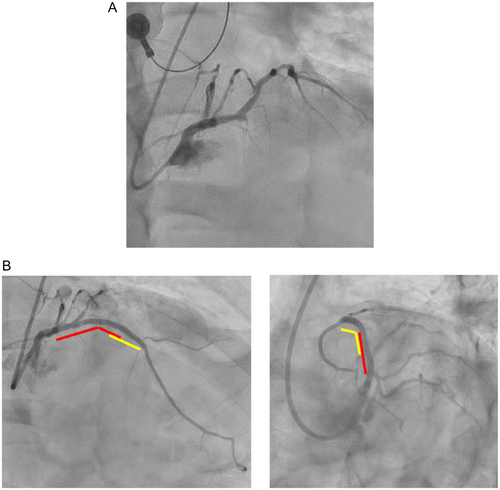
However, 1 month ago, he was transported to our hospital with chest pain and elevated cardiac biomarkers were noted. Emergent coronary angiography (CAG) revealed stent thrombosis (ST) of the LAD. He then underwent PCI using drug-coated-balloon (3.5 × 20 mm) application and a zotarolimus-eluting stent (ZES: Resolute Onyx 2.5 × 22 mm), and dual antiplatelet therapy had been restarted (Figure 2). At that time, there were no obvious signs of SF on either CAG or intravascular ultrasound (IVUS).
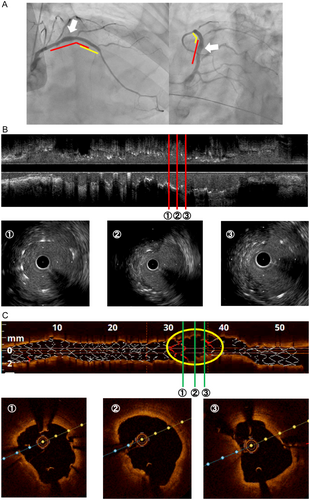
In the current episode, the patient again presented with chest pain and elevated cardiac enzymes, raising suspicion of recurrent ST. Emergent CAG was performed and revealed no obstructive coronary artery disease (Figure 3A). However, since there was a coronary artery aneurysm (CAA) at the BES, we then performed IVUS, in which stent-struts were not visible in the part complicated with CAA (Figure 3B). Since SF was suspected from IVUS findings, we additionally performed optical coherence tomography (OCT). OCT showed a transverse fracture with displacement complicated with CAA, allowing us to make a diagnosis of SF (Figure 3C).
We chose conservative therapy for the SF considering that there was no objective reason for the SF and no obstructive coronary artery disease. Initially, we continued dual antiplatelet therapy, but when a left ventricular thrombus was found in transthoracic echocardiography, we changed to single antiplatelet therapy and warfarin. We decided on careful follow-up for the CAA with imaging computer tomography angiography. He was discharged home after an uneventful hospital course.
3 Discussion
The incidence of DES fracture in current registries was reported to range from 1.7% to 2.6% and the incidence of SF for a SES (Cypher) was higher than for other types of DES [1]. Regarding BES, the SF of Nobori had been reported, however, there have been no previous reports of SF in Biofreedom [2].
The risk factors for SF are vessel angulation, use of SES, long stents, multiple stent layers and stent overlap, inflammation and hypersensitivity, stenting in the right coronary artery, heavy calcification, ostial bifurcational lesions, and stent overexpansion [3]. In the present patient, there were no obvious anatomical risk factors for SF, such as heavy calcification and ostial bifurcational lesions. The PCI was conducted successfully without long stents or stent overexpansion.
The structure of the stent may be a contributing factor. Although BES (Biofreedom) and BES (Nobori) differ in the presence or absence of a polymer, they share common characteristics such as relatively thick stent struts (Biofreedom: 112 μm, Nobori: 120 μm) and two inter-strut links. The SES (Cypher), which has been reported to have a high incidence of SF, also has a thick stent strut of 140 μm, suggesting that strut thickness may be a risk factor for SF. Furthermore, the small number of inter-ring connections results in low longitudinal strength, which may also contribute to an increased risk of SF [2]. This is because the continuous pulsation of the heart subjects the stent to various mechanical forces, including compression, torsion, bending, elongation, and shear stress. In the present case, a Type 3 SF was observed, suggesting that longitudinal stress may have played a role.
Although there was the possibility of a stent allergy because the material of the BES (Biofreedom) and ZES (Resolute Onyx) is cobalt, we were unable to determine whether there was a stent allergy because a patch test was not performed.
SF increases the risk of in-stent restenosis, ST, target lesion revascularization and major adverse cardiac events [3]. In the present patient, there were no obvious indications of SF with CAG and IVUS during the PCI for ST. The ST, however, may have been due to SF. Although there were no guidelines on diagnostic tools for the SF, high-resolution cine-angiography technology such as that used in StentBoost (Philips) and OCT should have been added to enhance visibility of stent struts [4, 5].
SFs are classified as isolated strut fractures (Type 1, single strut fracture; Type 2, incomplete transverse fracture) and complete fractures (Type 3, complete transverse fracture without displacement; Type 4, transverse fracture with displacement [6]. Clinically important adverse events, such as acute coronary syndromes, in-stent restenosis, and ST, mostly occur with Type 2, 3, and 4 fractures. Complete fractures are risk factors for CAA [7].
CAA caused by SF has a higher risk of rupture. While there are no guidelines for the management of CAA, surgical approaches, PCI, coil embolization, and medications are used [8, 9]. The CAA in this patient was a micro-aneurysm, one without dilatation exceeding 50% of the reference vessel. In the case of micro-aneurysms, PCI or surgical treatment can be avoided through careful follow-up using imaging modalities such as coronary computed tomography angiography [10]. Although the CAA in this patient had rapidly expanded in 1 month and might have been at higher risk of the rupture, there are no guidelines on follow-up periods for CAA. In the case of CAA with a rapidly expanding SF, short-term follow up for the CAA may be necessary.
4 Conclusion
We experienced the first case about SF of a Biofreedom (BES) complicated with a micro-CAA, where careful follow-up was needed for the CAA.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




