Impact of Elevated Preprocedural Left Ventricular Filling Pressure on Prognosis of Mild Paravalvular Regurgitation Following Transcatheter Aortic Valve Replacement
ABSTRACT
Background
Paravalvular regurgitation (PVR) following transcatheter aortic valve replacement (TAVR) is a complication linked to poor outcomes. The prognostic impact of mild PVR, particularly in patients with elevated preprocedural left ventricular (LV) filling pressure, remains uncertain.
Aims
This study aimed to assess the influence of elevated preprocedural LV filling pressure on mild PVR prognosis following TAVR.
Methods
This single-center, retrospective study analyzed consecutive patients with severe aortic stenosis who underwent TAVR, excluding those with moderate or severe PVR. Preprocedural LV filling pressure was evaluated using baseline E/A ratio, and patients were stratified into four groups based on E/A ratio (≤1 or >1) and PVR severity (none/trace or mild). The primary endpoint was cardiovascular death within 5 years.
Results
Among 904 patients, 466 had E/A ≤ 1 with none/trace PVR, 92 had E/A > 1 with none/trace PVR, 300 had E/A ≤ 1 with mild PVR, and 46 had E/A > 1 with mild PVR. Multivariable analysis identified E/A > 1 with mild PVR as an independent predictor of cardiovascular death (adjusted hazard ratio [HR]: 2.38; 95% confidence interval [CI]: 1.28–4.42; p < 0.01). In contrast, E/A > 1 with none/trace PVR (HR: 1.16, 95% CI: 0.66–2.03) and E/A ≤ 1 with mild PVR (HR: 1.33, 95% CI: 0.89–2.00) were not significant predictors compared to E/A ≤ 1 with none/trace PVR.
Conclusions
Elevated preprocedural LV filling pressure is independently associated with an increased risk of cardiovascular death in patients with mild PVR following TAVR.
Abbreviations
-
- AR
-
- aortic regurgitation
-
- AS
-
- aortic stenosis
-
- AV
-
- aortic valve
-
- BNP
-
- brain natriuretic peptide
-
- BSA
-
- body surface area
-
- CI
-
- confidence interval
-
- DT
-
- deceleration time
-
- eGFR
-
- estimated glomerular filtration rate
-
- HR
-
- hazard ratio
-
- IQR
-
- interquartile range
-
- LA
-
- left atrial
-
- LV
-
- left ventricular
-
- NYHA
-
- New York Heart Association
-
- PVR
-
- paravalvular regurgitation
-
- STS
-
- Society of Thoracic Surgeons
-
- TAVR
-
- transcatheter aortic valve replacement
-
- TR
-
- tricuspid regurgitation
-
- VARC-3
-
- Valve Academic Research Consortium 3
1 Introduction
Transcatheter aortic valve replacement (TAVR) has emerged as a viable alternative to surgical aortic valve replacement, even for patients with low-risk severe aortic stenosis (AS) [1, 2]. However, paravalvular regurgitation (PVR) remains a major complication associated with increased mortality after TAVR. Although moderate or severe PVR is well-established as a strong predictor of early and long-term mortality, the prognostic impact of mild PVR remains controversial [3, 4]. Variability in findings across studies may reflect differences in patient characteristics, and the prognostic factors for mild PVR have not been fully clarified.
Advances in device technology have reduced the incidence of moderate or severe PVR to less than 5% with new-generation prostheses [1, 2]. Therefore, identifying patients in whom mild PVR impacts prognosis has become clinically relevant.
Volume overload from PVR is a known contributor to poor outcomes after TAVR. However, the mechanisms through which mild regurgitation and its minor volume overload influence prognosis remain unclear. Severe AS causes chronic pressure overload, leading to concentric left ventricular (LV) hypertrophy and diastolic dysfunction. Moreover, many TAVR candidates are older adults with comorbidities, including overlapping heart failure from various etiologies [5]. Elevated preprocedural LV filling pressures due to AS or concomitant heart failure may render even mild PVR hemodynamically significant, potentially worsening clinical outcomes.
This study aimed to investigate the impact of elevated preprocedural LV filling pressure on clinical outcomes of mild PVR following TAVR.
2 Methods
2.1 Study Population
This single-center, retrospective, observational study included all patients with severe AS who underwent TAVR at Osaka University Hospital between October 2009 and December 2022. Patients who developed moderate or severe PVR after TAVR or underwent non-native aortic valve procedures (TAV-in-TAV or TAV-in-surgical bioprosthetic valve) were excluded. Additionally, patients with atrial fibrillation, prior permanent pacemaker implantation, severe mitral regurgitation, significant mitral stenosis, or previous mitral valve surgery were excluded, as these conditions interfere with the echocardiographic assessment of LV filling pressure. Patients without available echocardiographic data within 3 months before TAVR were also excluded. This study was approved by the Ethical Review Board Osaka University Hospital (Approval No. 23310). The requirement for informed consent was waived owing to the retrospective nature of the study.
2.2 TAVR Procedure
Indications for TAVR were determined by the heart team at Osaka University Hospital, following Japanese guidelines [6]. Prosthesis selection and access site determination were based on preprocedural CT angiography, analyzed using 3mensio Structural Heart (Pie Medical Imaging, Bilthoven, Netherlands). TAVR was performed under general or local anesthesia via transfemoral, alternative transarterial (including ascending aorta, subclavian, carotid, or iliac), or transapical approaches.
2.3 Data Collection and Clinical Endpoint
Patient data, including age, sex, body mass index, comorbidities, New York Heart Association (NYHA) functional classification, the Society of Thoracic Surgeons (STS) predicted risk of mortality, and laboratory values, were retrospectively collected from clinical records at the time of TAVR admission. Clinical follow-up was conducted through visits, letters, or telephone contact at 1, 6, and 12 months post-TAVR, then annually thereafter for up to 5 years. The primary endpoint was cardiovascular death within 5 years after TAVR, as defined by the Valve Academic Research Consortium 3 (VARC-3) criteria [7].
2.4 Assessment of Echocardiography
Transthoracic echocardiography was performed at baseline within 3 months before TAVR and 1, 6, and 12 months after the procedure. Echocardiographic variables were analyzed by experienced sonographers following the guidelines of the American Society of Echocardiography and the European Association of Cardiovascular Imaging [8, 9]. Transmitral flow parameters, including peak velocity in early diastole (E-wave) and late diastole (A-wave), E/A ratio, and deceleration time (DT) of the E-wave, were measured using pulsed-wave Doppler in the apical 4-chamber view. Early diastolic annular velocity (e') of the lateral and septal mitral annulus was assessed using tissue Doppler imaging, and the averaged E/e' ratio was calculated by dividing the E velocity by the mean of the septal and lateral e' velocities. The left atrial (LA) volume index was measured using the biplane method and indexed to body surface area (BSA). Peak aortic valve (AV) velocity, mean AV gradient, and peak tricuspid regurgitation (TR) velocity were measured using continuous-wave Doppler. AV area was calculated using the continuity equation. LV ejection fraction was assessed using the Teichholz method or modified Simpson's rule if abnormal LV wall motion was present. LV mass was calculated using the cube formula and indexed to BSA. The severity of PVR was evaluated 1 month after TAVR via echocardiography using a three-class grading system according to the VARC-3 criteria [7].
2.5 Estimation of LV Filling Pressure
Preprocedural LV filling pressure was estimated using the baseline E/A ratio. An E/A ratio > 1, indicating a pseudo-normalized or restrictive filling pattern, was considered indicative of elevated LV filling pressure [10, 11]. Patients were classified into four groups based on the baseline E/A ratio (≤1 or >1) and the severity of residual PVR (none/trace or mild). Baseline brain natriuretic peptide (BNP) levels were also used as an alternative indicator of LV filling pressure [12], and patients were grouped according to the median BNP values of the study cohort and whether PVR was none/trace or mild.
2.6 Statistical Analysis
Continuous variables are presented as mean ± SD or median with interquartile range (IQR); in contrast, categorical variables are expressed as numbers and percentages. Comparisons of continuous variables were performed using the one-way analysis of variance or the Kruskal–Wallis rank sum test, depending on the distribution of the variables. Categorical variables were compared using the chi-squared test. Time-to-event variables are presented as Kaplan–Meier estimates, with comparisons among the four groups classified by pre-E/A ratio and residual PVR using the log-rank test. Univariable and multivariable Cox proportional hazard models were used to assess the risk of cardiovascular death, with results expressed as hazard ratio (HR) and 95% confidence interval (CI). The multivariable models were adjusted for relevant variables, including age, sex, body mass index, NYHA functional class III or IV, STS score, chronic obstructive pulmonary disease, hypertension, diabetes, hemoglobin, estimated glomerular filtration rate (eGFR), serum albumin, LV ejection fraction, mean AV gradient, non-transfemoral approach, and use of a balloon-expandable valve (BEV) [3, 4, 13]. Similarly, in the four groups classified by baseline BNP levels and residual PVR, cardiovascular mortality events after TAVR were represented by Kaplan–Meier curves and compared using the log-rank test. Univariable and multivariable Cox proportional hazards models were also performed. Statistical significance was defined as a p < 0.05. All statistical analyses were performed using R software version 4.4.1 (The R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Study Population
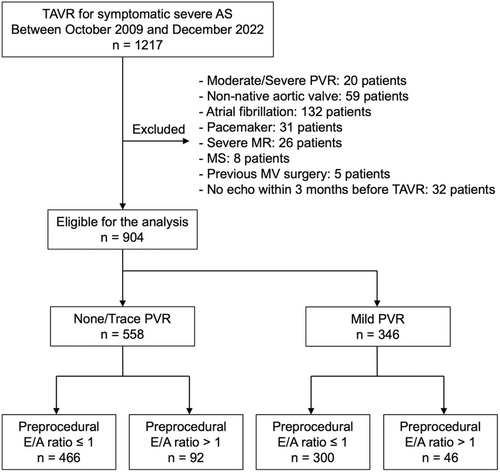
The study flow diagram is shown in Figure 1. Among 1217 consecutive patients who underwent TAVR between October 2009 and December 2022, 939 patients were eligible for this analysis. They were categorized based on their baseline E/A ratio and residual PVR: E/A ≤ 1 with none/trace PVR (n = 466), E/A > 1 with none/trace PVR (n = 92), E/A ≤ 1 with mild PVR (n = 300), and E/A > 1 with mild PVR (n = 46). The median follow-up duration was 3.1 years (IQR: 1.9–5.0 years). The baseline and procedural characteristics of the study population are detailed in Table 1. In the overall cohort, the mean age was 83 years, and 62% of the patients were female. Patients with the E/A > 1 were more likely to be male and receiving hemodialysis, and they had higher STS scores. Preprocedural laboratory data showed higher BNP levels and lower hemoglobin, eGFR, and serum albumin levels in the E/A > 1 groups. The baseline echocardiographic parameters are summarized in Table 2. LV ejection fraction was lower, and LV mass index was higher in the E/A > 1 groups compared to that in the E/A ≤ 1 groups. Other echocardiographic measures of LV filling pressure, including DT, peak TR velocity, LA volume index, and E/e’ ratio, were also higher in the E/A > 1 group.
| None/Trace PVR | Mild PVR | ||||
|---|---|---|---|---|---|
| E/A ≤ 1 (n = 466) | E/A > 1 (n = 92) | E/A ≤ 1 (n = 300) | E/A > 1 (n = 46) | p value | |
| Age, yrs | 83.4 ± (5.3) | 82.3 ± 6.9 | 83.9 ± 6.1 | 83.1 ± 7.0 | 0.16 |
| Male | 149 (32.0) | 37 (40.2) | 125 (41.7) | 22 (47.8) | 0.01 |
| Body mass index, kg/m2 | 22.4 ± 3.7 | 22.5 ± 3.7 | 22.0 ± 3.4 | 21.6 ± 2.8 | 0.24 |
| Hypertension | 379 (81.3) | 72 (78.3) | 232 (77.3) | 30 (65.2) | 0.06 |
| Diabetes mellitus | 148 (31.8) | 35 (38.0) | 82 (27.3) | 13 (28.3) | 0.23 |
| Dyslipidemia | 267 (57.3) | 56 (60.9) | 159 (53.0) | 22 (47.8) | 0.32 |
| Hemodialysis | 44 (9.4) | 18 (19.6) | 25 (8.3) | 8 (17.4) | 0.01 |
| COPD | 82 (17.6) | 13 (14.1) | 47 (15.7) | 13 (28.3) | 0.16 |
| Peripheral artery disease | 97 (20.8) | 28 (30.4) | 66 (22.0) | 12 (26.1) | 0.22 |
| Prior MI | 38 (8.2) | 7 (7.6) | 24 (8.0) | 6 (13.0) | 0.69 |
| Prior PCI | 104 (22.3) | 28 (30.4) | 57 (19.0) | 12 (26.1) | 0.12 |
| Prior CABG | 34 (7.3) | 13 (14.1) | 17 (5.7) | 5 (10.9) | 0.05 |
| STS score, % | 6.3 [4.4, 9.4] | 8.0 [5.4, 11.0] | 6.1 [4.4, 8.8] | 6.8 [4.8, 13.3] | < 0.01 |
| NYHA class III or IV | 173 (37.1) | 41 (44.6) | 103 (34.3) | 19 (41.3) | 0.32 |
| Laboratory values | |||||
| BNP, pg/ml | 149.5 [77.5, 348.0] | 390.4 [195.9, 883.5] | 161.7 [89.7, 364.6] | 466.5 [217.9, 1173.8] | < 0.01 |
| Hemoglobin, g/dl | 11.5 ± 1.7 | 11.1 ± 1.5 | 11.4 ± 1.7 | 10.9 ± 2.0 | 0.04 |
| eGFR, ml/min/1.73 m2 | 49.6 ± 22.6 | 40.6 ± 23.1 | 50.8 ± 24.0 | 39.0 ± 22.8 | < 0.01 |
| Albumin, g/dl | 3.7 ± 0.5 | 3.6 ± 0.5 | 3.8 ± 0.4 | 3.6 ± 0.5 | 0.01 |
| Procedural characteristics | |||||
| Balloon-expandable valve | 286 (61.4) | 68 (73.9) | 150 (50.0) | 23 (50.0) | < 0.01 |
| Valve with an outer skirt | 218 (46.8) | 46 (50.0) | 165 (55.0) | 31 (67.4) | 0.02 |
| Balloon pre-dilatation | 311 (67.0) | 59 (64.8) | 209 (69.7) | 31 (67.4) | 0.81 |
| Balloon post-dilatation | 96 (20.8) | 11 (12.0) | 90 (30.2) | 16 (35.6) | < 0.01 |
| Transfemoral approach | 342 (73.4) | 65 (70.7) | 247 (82.3) | 35 (76.1) | 0.02 |
- Note: Values are mean ± SD, median [interquartile range], or n (%)
- Abbreviations: AR = aortic regurgitation; AV = aortic valve; BNP = brain natriuretic peptide; CABG = coronary artery bypass graft; COPD = chronic obstructive pulmonary disease; eGFR = estimated glomerular filtration rate; MI = myocardial infarction; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; PVR = paravalvular regurgitation; STS = Society of Thoracic Surgeons.
| None/Trace PVR | Mild PVR | ||||
|---|---|---|---|---|---|
| E/A ≤ 1 (n = 466) | E/A > 1 (n = 92) | E/A ≤ 1 (n = 300) | E/A > 1 (n = 46) | p value | |
| LV geometry and systolic function | |||||
| LV end-diastolic diameter, mm | 44.8 ± 6.8 | 48.6 ± 7.6 | 43.7 ± 6.4 | 47.5 ± 6.3 | <0.01 |
| LV end-systolic diameter, mm | 29.1 ± 7.4 | 33.6 ± 9.1 | 28.2 ± 6.8 | 33.3 ± 7.8 | <0.01 |
| Intraventricular septum diameter, mm | 11.2 ± 2.0 | 11.3 ± 1.7 | 11.6 ± 1.7 | 11.3 ± 1.7 | 0.04 |
| LV posterior wall diameter, mm | 10.9 ± 1.8 | 11.2 ± 1.8 | 11.3 ± 1.8 | 11.0 ± 1.7 | 0.03 |
| LV ejection fraction, % | 64.3 ± 11.9 | 58.2 ± 14.0 | 65.0 ± 11.7 | 56.6 ± 14.5 | <0.01 |
| LV mass index, g/m2 | 123.0 ± 34.1 | 139.5 ± 33.3 | 123.4 ± 33.2 | 131.0 ± 28.2 | <0.01 |
| LV diastolic function | |||||
| E-wave velocity, m/s | 0.7 ± 0.2 | 1.1 ± 0.3 | 0.7 ± 0.2 | 1.0 ± 0.3 | <0.01 |
| A-wave velocity, m/s | 1.2 ± 0.3 | 0.8 ± 0.3 | 1.1 ± 0.3 | 0.7 ± 0.2 | <0.01 |
| E/A ratio | 0.6 ± 0.2 | 1.5 ± 0.6 | 0.6 ± 0.2 | 1.6 ± 0.6 | <0.01 |
| Deceleration time, ms | 271.3 ± 96.4 | 216.8 ± 98.4 | 275.9 ± 96.4 | 202.6 ± 78.1 | <0.01 |
| Septal e’ velocity, cm/s | 3.5 ± 1.2 | 3.9 ± 1.2 | 3.4 ± 1.1 | 3.8 ± 1.4 | 0.01 |
| Lateral e’ velocity, cm/s | 4.7 ± 1.8 | 4.8 ± 1.5 | 4.6 ± 1.6 | 5.1 ± 2.0 | 0.32 |
| Average E/e’ ratio | 18.9 ± 8.9 | 27.9 ± 13.4 | 18.7 ± 9.3 | 25.1 ± 11.7 | <0.01 |
| LA volume index, ml/m2 | 48.4 ± 15.6 | 61.7 ± 15.9 | 50.0 ± 27.7 | 62.1 ± 18.6 | <0.01 |
| Hemodynamics | |||||
| AV area, cm2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.62 |
| AV peak velocity, m/s | 4.4 ± 0.7 | 4.4 ± 0.9 | 4.6 ± 0.7 | 4.5 ± 0.7 | 0.11 |
| AV mean gradient, mmHg | 48.9 ± 16.2 | 49.6 ± 19.6 | 52.4 ± 18.3 | 50.7 ± 17.9 | 0.06 |
| TR peak velocity, m/s | 2.5 ± 0.3 | 2.9 ± 0.5 | 2.5 ± 0.4 | 3.0 ± 0.5 | <0.01 |
| Moderate or severe AR | 22 (4.7) | 10 (10.9) | 11 (3.7) | 3 (6.5) | 0.05 |
| Moderate or severe TR | 8 (1.7) | 1 (1.1) | 2 (0.7) | 3 (6.5) | 0.03 |
- Note: Values are mean ± SD or n (%).
- Abbreviations: AR = aortic regurgitation, AV = aortic valve, LA = left atrial, LV = left ventricular, PVR = paravalvular regurgitation, TR = tricuspid regurgitation.
3.2 Procedural Characteristics
The TAVR procedure was successfully performed in all patients. Mild PVR occurred less frequently in patients receiving BEVs than in those receiving self-expandable valves (SEVs). While valves with an outer skirt did not significantly reduce the incidence of mild PVR compared to those without an outer skirt, balloon post-dilatation was performed less frequently in valves with an outer skirt (Table S1).
3.3 Baseline E/A Ratio and PVR
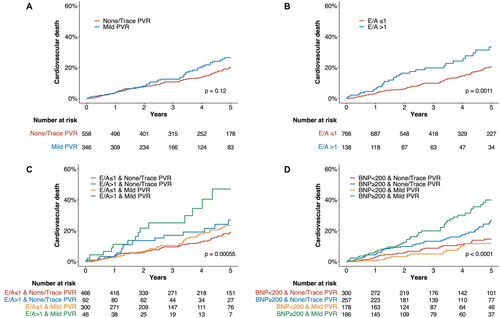
Figure 2 presents the Kaplan–Meier curves for cardiovascular death. No significant difference in cardiovascular death was observed between the none/trace PVR and mild PVR groups (Figure 2A). However, the baseline E/A > 1 group showed a significantly higher prevalence of cardiovascular death than did the E/A ≤ 1 group (Figure 2B). The cumulative incidence of cardiovascular death was highest in the E/A > 1 with mild PVR group (overall log-rank p < 0.01, Figure 2C). In a multivariable Cox proportional hazard model, the E/A > 1 with mild PVR was identified as an independent predictor of cardiovascular death (adjusted HR: 2.38; 95% CI: 1.28–4.42; p < 0.01); in contrast, E/A > 1 with none/trace PVR and E/A ≤ 1 with mild PVR were not significant predictors, using E/A ≤ 1 with none/trace PVR as the reference (Table 3).
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| E/A ratio and PVR | ||||
| E/A ≤ 1 with none/trace PVR | Reference | Reference | ||
| E/A > 1 with none/trace PVR | 1.62 (0.95−2.78) | 0.08 | 1.16 (0.66−2.03) | 0.61 |
| E/A ≤ 1 with mild PVR | 1.21 (0.82−1.80) | 0.33 | 1.33 (0.89−2.00) | 0.17 |
| E/A > 1 with mild PVR | 3.17 (1.77−5.68) | <0.01 | 2.38 (1.28−4.42) | <0.01 |
| BNP and PVR | ||||
| BNP < 200 with none/trace PVR | Reference | Reference | ||
| BNP ≥ 200 with none/trace PVR | 1.84 (1.16−2.90) | <0.01 | 1.14 (0.68−1.91) | 0.62 |
| BNP < 200 with mild PVR | 0.73 (0.38−1.43) | 0.36 | 0.76 (0.39−1.50) | 0.43 |
| BNP ≥ 200 with mild PVR | 2.94 (1.85−4.67) | <0.01 | 2.11 (1.26−3.53) | <0.01 |
- Abbreviations: BNP = brain natriuretic peptide, LV = left ventricular, PVR = paravalvular regurgitation, Ref. = reference, TAVR = transcatheter aortic valve replacement.
3.4 Baseline BNP Levels and PVR
The Kaplan–Meier curves stratified by preprocedural BNP levels and PVR severity are shown in Figure 2D. The cutoff value for BNP was set at 200 pg/mL. Similar to the E/A ratio classification, the cumulative incidence of cardiovascular death was highest in the high BNP with mild PVR group (overall log-rank p < 0.01). In a multivariable model, high BNP with mild PVR was also found to be an independent predictor of cardiovascular death (adjusted HR: 2.11; 95% CI: 1.26–3.53; p < 0.01); in contrast, high BNP with none/trace PVR and low BNP with mild PVR were not significant predictors, using low BNP with none/trace PVR as the reference (Table 3).
3.5 Postprocedural Assessment
Serial changes in echocardiographic parameters and BNP levels after TAVR are presented in Table S2. BNP levels declined after TAVR in all groups. In many patients with a baseline E/A > 1, the E/A ratio decreased following TAVR. However, the E/A ratio tended to remain > 1 in those with mild PVR compared to those with none/trace PVR, although the difference was not statistically significant.
4 Discussion
This study demonstrated that mild PVR in patients with elevated LV filling pressure at baseline was associated with an increased risk of cardiovascular death. Specifically, mild PVR in patients with a baseline E/A ratio > 1 or BNP levels ≥ 200 pg/mL was identified as an independent predictor of cardiovascular death.
PVR is a significant complication of TAVR and has been recognized as a predictor of cardiac death related to advanced heart failure [14]. Although previous large-scale studies have consistently shown that moderate or severe PVR is associated with both short- and long-term mortality, the prognostic significance of mild PVR remains controversial [3, 4]. In patients with AS, chronic pressure overload induces LV remodeling, resulting in concentric hypertrophy and increased myocardial stiffness. A stiff, normal-sized LV is often unable to adequately compensate for the volume overload induced by PVR following TAVR. This failure to compensate further increases LV end-diastolic pressure and worsens outcomes [15]. This mechanism suggests that the prognostic impact of PVR is not solely determined by the degree of regurgitation but is also influenced by pre-existing diastolic dysfunction and elevated LV filling pressure.
In addition to the pathophysiology of AS itself, older adult patients eligible for TAVR often present with various comorbidities, such as hypertension, diabetes, or chronic kidney disease, all of which contribute to heart failure with diastolic dysfunction. Concomitant coronary artery disease is also common in these patients, and, in some cases, the etiology of AS is related to cardiac amyloidosis [5]. Given this complex clinical background, even mild PVR may adversely affect the prognosis of TAVR patients with elevated LV filling pressures due to advanced AS and other comorbidities. In the present study, mild PVR alone was not significantly associated with cardiovascular death. However, the combination of mild PVR and elevated preprocedural LV filling pressure emerged as an independent predictor of worse outcomes, even after adjusting for potential confounders. In contrast, elevated LV filling pressure alone was associated with increased mortality, consistent with findings from prior studies [16, 17]. A noteworthy finding was that elevated LV filling pressure did not correlate significantly with mortality when combined with none/trace PVR. These observations suggest that mild PVR substantially increases the risk of future cardiovascular death, specifically in patients with elevated preprocedural LV filling pressure. Conversely, patients with a compliant LV capable of tolerating mild PVR or those with reduced LV compliance but no residual PVR may experience better outcomes. The association between elevated baseline BNP levels and residual PVR further supports this hypothesis. On the other hand, this study did not fully elucidate the mechanisms linking mild PVR to adverse outcomes in patients with elevated LV filling pressure. However, postprocedural echocardiography revealed that the E/A ratio following TAVR tended to remain > 1 in patients with mild PVR. This suggests that mild PVR may hinder the expected decline in filling pressure after AS resolution. Further prospective studies are needed to clarify these mechanisms.
The findings of this study corroborate previous analyses conducted on smaller patient cohorts. In a retrospective study of 146 patients with AS with diastolic dysfunction [18], those with severe diastolic dysfunction who developed mild or greater aortic regurgitation (AR) following TAVR experienced increased mortality compared with other patients. However, the study did not clarify the prognostic impact of baseline diastolic dysfunction on mild AR alone. In another retrospective study of 418 patients undergoing TAVR [19], a preprocedural DT of E-wave < 160 ms, typically seen in cases of restrictive filling, was independently associated with mortality among patients with mild AR after TAVR. Given that the population in these previous studies likely had more severe diastolic impairment compared to our cohort, our findings are novel in suggesting that even mild elevated LV filling pressure can worsen prognosis when PVR occurs. The E/A ratio used in this analysis is known to correlate with LV end-diastolic pressure [20]. Although a normal E/A ratio > 1 is common in younger individuals with normal diastolic function, this study cohort was older, had severe AS, and exhibited other indicators of elevated LV end-diastolic pressures, such as increased E/e’ ratio, LA volume index, or TR velocity. Therefore, the E/A ratio > 1 in our cohort is reasonably considered indicative of elevated LV end-diastolic pressure. Furthermore, future research incorporating other parameters related to both systolic and diastolic function may improve prognostic accuracy.
These findings have clinical significance as they may guide therapeutic strategies for managing PVR. In this study, BEVs were associated with a lower incidence of mild PVR compared to SEVs. In contrast, the newer-generation valves with an outer skirt did not significantly reduce mild PVR compared to the previous-generation valves lacking an outer skirt. However, balloon post-dilatation was performed less frequently in the newer-generation valves, suggesting that the outer skirt may have contributed to some degree of PVR reduction. This also implies that post-dilatation remains a viable option for controlling PVR to less than mild when necessary. These findings suggest that using BEVs or performing balloon post-dilatation may be beneficial for patients with elevated LV filling pressure. In contrast, given these techniques also carry risks, including annulus rupture, coronary artery occlusion, conduction disturbance, and embolism [21, 22], aggressively reducing PVR to less than mild may not be necessary for patients without elevated filling pressure. Identifying patients for whom mild PVR could worsen prognosis is crucial for minimizing complications and improving TAVR outcomes. The E/A ratio and BNP levels are practical tools for preoperative risk stratification. Future research should validate these findings through prospective studies and aim to establish them as standard methodologies for clinical practice.
This study has some limitations. First, it was conducted at a single center with a relatively modest sample size; however, it is larger than similar previous studies. Second, the study cohort excluded patients with atrial fibrillation, prior pacemaker implantation, and significant mitral valve disease. Third, the interpretation of postprocedural echocardiographic findings is limited by missing data and potential survival bias. Finally, cardiovascular events other than mortality, such as heart failure hospitalizations, were not assessed because of limitations in medical records data collection. Further prospective studies considering additional clinical outcomes will be needed.
5 Conclusion
Elevated preprocedural LV filling pressure is independently associated with cardiovascular death in patients with mild PVR following TAVR. These findings could help identify patients whose prognosis may be adversely affected by mild PVR and guide therapeutic strategies in TAVR. Future studies should validate these results through prospective trials and explore additional clinical outcomes, such as heart failure hospitalizations, to further refine patient management strategies.
Acknowledgments
The authors thank all Osaka University Hospital Heart Team members who contributed to data acquisition and cooperated with the TAVR procedure.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




