Fractional Flow Reserve Directed Percutaneous Coronary Intervention Optimization Using High-Definition Intravascular Ultrasound in Non-ST-Segment Elevation Acute Coronary Syndrome Versus Chronic Coronary Syndrome
ABSTRACT
Background
Intravascular ultrasound (IVUS)-guided optimization of suboptimal fractional flow reserve (FFR) following percutaneous coronary intervention (PCI) results in a significant increase in both post-PCI FFR and minimal lumen and stent areas (MLA and MSA, respectively). However, the impact of clinical presentation with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) versus chronic coronary syndrome (CCS) on the efficacy of PCI optimization remains unknown.
Methods
This was a prespecified subgroup analysis of the FFR REACT trial comparing IVUS-guided PCI optimization versus no further treatment in 291 patients with a post-PCI FFR < 0.90. Post-PCI physiology and pre optimization IVUS findings were compared between patients presenting with NSTE-ACS versus CCS, as well as optimization strategy, final FFR and IVUS findings.
Results
Out of 291 patients, 130 (44.7%) presented with NSTE-ACS. Median post-PCI FFR was similar in patients with NSTE-ACS and CCS (0.85 for both, p = 0.55). Pre optimization IVUS findings did not differ significantly between both groups and subsequent optimization strategy was comparable (p = 0.71). In both NSTE-ACS and CCS, optimization resulted in a significant increase (p < 0.01 for all) of similar magnitude in median FFR (0.02 vs. 0.03, p = 0.80), MLA (0.37 vs. 0.50 mm2, p = 0.46) and MSA (0.29 vs. 0.32 mm2, p = 0.61), respectively. The clinical impact of IVUS-guided optimization on 2-year target vessel failure showed no signs of heterogeneity based on clinical presentation (interaction p = 0.36).
Conclusions
In patients undergoing FFR-directed IVUS-guided optimization, post-PCI FFR, pre optimization IVUS findings and optimization strategy did not differ significantly between patients presenting with either NSTE-ACS or CCS, with comparable improvements in FFR, MLA and MSA.
1 Introduction
A growing body of evidence has demonstrated a significant association between impaired post percutaneous coronary intervention (PCI) fractional flow reserve (FFR) and future adverse clinical events [1-7]. Several observational studies and one randomized clinical trial subsequently demonstrated that post-PCI FFR can be increased by additional postdilatation or stent placement [8-10]. Optimization in these studies was typically directed by FFR alone, precluding identification of the anatomical substrate for suboptimal post-PCI FFR [8-10]. In contrast to FFR, intravascular ultrasound (IVUS) facilitates a precise interpretation of underexpansion, stenting-related complications and residual focal lesions, allowing targeted PCI optimization in patients with low post-PCI FFR [11].
In the recently published randomized FFR REACT trial we demonstrated that IVUS-guided PCI optimization in patients with a post-PCI FFR < 0.90 resulted in a significant improvement of final post-PCI FFR, increased luminal and stent areas, and a trend toward lower rates of target vessel revascularization (TVR) at 1 year [12].
Given the differences in pathophysiological disease mechanisms between patients presenting with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) and those presenting with chronic coronary syndrome (CCS), it remains unknown how post-PCI physiological assessment and intravascular imaging findings may impact the feasibility of IVUS-guided optimization as directed by post-PCI FFR in both clinical cohorts. Therefore, the aim of this study was to compare physiology and IVUS findings, as well as optimization strategy and clinical outcome in relation to IVUS-guided optimization, in patients presenting with either NSTE-ACS or CCS.
2 Methods
2.1 Study Design and Study Population
This is a prespecified subgroup analysis of the investigator-initiated, single-center, randomized FFR REACT trial (NL6523). Between October 31, 2017, and April 22, 2020, 291 patients with a post-PCI FFR < 0.90 in at least 1 vessel were randomized to receive IVUS-guided optimization versus no further treatment. To fulfill the aim of this prespecified subgroup analysis, included patients were divided into two groups based on their clinical presentation: (1) NSTE-ACS, including both unstable angina and non-ST-segment elevation myocardial infarction, or (2) CCS.
Patients presenting with ST-segment elevation myocardial infarction or cardiogenic shock/hemodynamic instability, a target vessel distal reference diameter < 2.25 mm (precluding post-PCI FFR measurement with a microcatheter), or undergoing PCI without stent implantation, were excluded from the trial as described previously [13]. The study protocol was approved by the local ethics committee on October 26, 2017, and the study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided informed consent before any study-related procedures.
2.2 Study Procedures and Study Materials
Details on study procedures have been previously published [12, 13]. In brief, after angiographic confirmation of successful PCI (<30% stenosis, TIMI flow grade 3), post-PCI and FFR measurements were performed using a dedicated microcatheter (Navvus, ACIST Medical Systems Inc.). Measurements were performed at least 20 mm distal to the most distal stent edge. Maximal hyperemia was achieved by an intravenous infusion of adenosine at a rate of 140 μg/kg/min through an antecubital vein. Measurements were repeated when the pressure drift was >0.02.
Patients were randomized if the post-PCI FFR value was below 0.90. Randomization to the control arm led to the end of the procedure (i.e., standard of care). In patients randomized to the IVUS-guided optimization arm, IVUS was performed by an automated pullback (Kodama HD-IVUS catheter, ACIST Medical Systems Inc) at 2.5 mm/s (24 frames/mm) starting from the same location as the FFR measurement. PCI optimization was performed according to a study specific, dedicated optimization protocol [12, 13]. When PCI optimization was performed, both FFR and IVUS were repeated to evaluate the treatment results. Patients were discharged on guideline-recommended medical therapy.
2.3 Offline FFR and IVUS Analysis
All FFR tracings and IVUS pullbacks were analyzed by the Erasmus University Medical Center academic core lab. A detailed description, including all IVUS definitions, has been published before [12, 13].
The segment target to optimization was identified in each pullback to directly compare the pre- and postoptimization luminal and stent areas in three optimization scenarios: scenario 1, “postdilatation only” in which the region of interest included the stented segment +5 mm reference segments distal and proximal; scenario 2, “additional stenting without optimization of the initial stent,” which included the newly placed stent +5 mm reference segments distal and proximal; and scenario 3, “additional stenting in combination with initial stent optimization,” which included both stents +5 mm reference segments distal and proximal [12].
2.4 Clinical Follow-Up
The follow-up data was collected by study personnel unaware of post-PCI FFR values and treatment allocation through telephone calls and outpatient clinic visits. If the patient reported an event, additional information was obtained from electronic patient records, referring hospitals or general practitioners.
2.5 Clinical Endpoints, Definitions and Event Adjudication
The primary endpoint was target vessel failure (TVF) at 2 years, a composite of cardiac death, spontaneous target vessel (Q-wave or non-Q-wave) myocardial infarction, and clinically driven TVR. The target vessel was defined as the vessel that was interrogated during the study-specific interventions.
Secondary endpoints included the individual components of the primary endpoint, as well as clinically driven target lesion revascularization, all-cause mortality, any spontaneous myocardial infarction, and any revascularization.
According to predefined definitions, events were adjudicated by an independent clinical event committee unaware of the patient's post-PCI FFR values and study arm allocation [13].
2.6 Statistical Analysis
Continuous variables are presented as medians (25th−75th percentiles). Categorical variables are shown as counts and percentages.
Normality of continuous variables was evaluated by the Shapiro-Wilk test. Differences between the NSTE-ACS and CCS group were assessed using the Student's T-test and Mann−Whitney U test (according to the distribution) for continuous patient-level variables and linear mixed models with random intercepts for continuous vessel-level variables to correct for clustering of vessels within patients. Categorical variables were compared using the Pearson's χ2 test or Fisher's exact test (as appropriate) at patient level and generalized linear mixed models with random intercepts at vessel level. For comparison of the final physiology and IVUS findings (following PCI optimization), clustering of vessels within patients was considered negligible, since only one patient had two vessels examined. Therefore, standard statistical tests were applied. Changes in post-PCI FFR, minimal lumen area (MLA) and minimal stent area (MSA) following optimization were assessed with the paired t-test or Wilcoxon signed-rank test, as appropriate.
For the survival analysis of the 2-year clinical endpoints, patients were censored at the day of the event of interest, at the last day of contact or at 730 days, whichever occurred first. For the composite primary endpoint, the first occurring event was counted. The Kaplan-Meier estimator was used to calculate event probabilities, and the log-rank test was applied to test for differences in survival time distributions between IVUS-guided optimization versus standard of care. Univariable hazard ratios (HRs) including 95% confidence intervals (CIs) were derived from Cox proportional hazard regression models. To determine whether the impact of IVUS-guided optimization on TVF was differential for NSTE-ACS versus CCS, interaction was assessed using Cox proportional hazard regression analysis. For this purpose, product terms were added to the model consisting of optimization and clinical presentation. In addition, TVF event probabilities between the two study arms and both clinical cohorts were compared using the log-rank test. No interaction assessment and log-rank tests were performed for the secondary endpoints due to limited number of events in small sample sized subgroups.
A 2-sided p value was considered to be statistically significant, and statistical tests were performed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp) and R software, version 4.1.0, (R Core Team 2021, packages: glme, nlme).
3 Results
3.1 Study Population, Baseline and Procedural Characteristics
Baseline and procedural characteristics are presented in Tables 1 and 2. Out of the 291 patients with a post-PCI FFR < 0.90, 130 (44.7%) patients (135 vessels) presented with NSTE-ACS and 161 (55.3%) patients (174 vessels) presented with CCS.
| Patient-level analysis | NSTE-ACS (n = 130) | CCS (n = 161) | p* | ||||
|---|---|---|---|---|---|---|---|
| All (n = 130) | IVUS (n = 56) | Control (n = 74) | All (n = 161) | IVUS (n = 89) | Control (n = 72) | ||
| Age (years) | 66.0 (56.0−75.3) | 65.0 (55.3−73.0) | 66.0 (56.0−77.3) | 66.0 (59.5−73.0) | 66.0 (60.5−71.0) | 67.0 (57.5−73.0) | 0.98 |
| Male | 102 (78.5) | 45 (80.4) | 57 (77.0) | 133 (82.6) | 78 (87.6) | 55 (76.4) | 0.37 |
| Hypertension | 93 (71.5) | 37 (66.1) | 56 (75.7) | 115 (71.4) | 64 (71.9) | 51 (70.8) | 0.98 |
| Hypercholesterolemia | 76 (58.5) | 37 (66.1) | 39 (52.7) | 113 (70.2) | 63 (70.8) | 50 (69.4) | 0.037 |
| Diabetes, insulin dependent | 7 (5.4) | 3 (5.4) | 4 (5.4) | 17 (10.6) | 11 (12.4) | 6 (8.3) | 0.11 |
| Smoking history | 70 (53.8) | 28 (50.0) | 42 (56.8) | 77 (47.8) | 45 (50.6) | 32 (44.4) | 0.31 |
| Prior myocardial infarction | 30 (23.1) | 14 (25.0) | 16 (21.6) | 38 (23.6) | 22 (24.7) | 16 (22.2) | 0.92 |
| Prior PCI | 34 (26.2) | 14 (25.0) | 20 (27.0) | 58 (36.0) | 33 (37.1) | 25 (34.7) | 0.07 |
| Prior CABG | 6 (4.6) | 3 (5.4) | 3 (4.1) | 6 (3.7) | 4 (4.5) | 2 (2.8) | 0.71 |
| Prior stroke | 15 (11.5) | 10 (17.9) | 5 (6.8) | 11 (6.8) | 7 (7.9) | 4 (5.6) | 0.16 |
| Peripheral artery disease | 15 (11.5) | 5 (8.9) | 10 (13.5) | 14 (8.7) | 8 (9.0) | 6 (8.3) | 0.42 |
| Atrial fibrillation | 5 (3.8) | 2 (3.6) | 3 (4.1) | 10 (6.2) | 8 (9.0) | 2 (2.8) | 0.36 |
| eGFR (ml/min) | 84.0 (67.0−94.0) | 83.5 (68.8−95.0) | 84.0 (63.5−94.0) | 84.0 (68.8−93.0) | 86.0 (71.0−93.0) | 83.0 (64.0−93.0) | 0.92 |
| Clinical presentation | NA | ||||||
| Stable angina | 0 (0.0) | 0 (0.0) | 0 (0.0) | 161 (100.0) | 89 (100.0) | 72 (100.0) | |
| Unstable angina | 43 (33.1) | 17 (30.4) | 26 (35.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| NSTEMI | 87 (66.9) | 39 (69.6) | 48 (64.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
- Note: Values are presented as median (25th−75th percentiles) or n (%).
- Abbreviations: CABG = coronary artery bypass graft surgery, CCS = chronic coronary syndrome, eGFR = estimated glomerular filtration rate, NA = not applicable, NSTE-ACS = non-ST-segment elevation acute coronary syndrome, NSTEMI = non-ST-segment elevation myocardial infarction, PCI = percutaneous coronary intervention.
- * p value for all NSTE-ACS versus all CCS.
| A. Vessel-level analysis* | NSTE-ACS (n = 135) | CCS (n = 174) | p** | ||||
|---|---|---|---|---|---|---|---|
| All (n = 135) | IVUS (n = 57) | Control (n = 78) | All (n = 174) | IVUS (n = 95) | Control (n = 79) | ||
| Target vessel | 0.32 | ||||||
| RCA | 17 (12.6) | 10 (17.5) | 7 (9.0) | 29 (16.7) | 15 (15.8) | 14 (17.7) | |
| LM | 1 (0.7) | 1 (1.8) | 0 (0.0) | 2 (1.1) | 1 (1.1) | 1 (1.3) | |
| LAD | 103 (76.3) | 39 (68.4) | 64 (82.1) | 126 (72.4) | 70 (73.7) | 56 (70.9) | |
| LCx | 13 (9.6) | 7 (12.3) | 6 (7.7) | 17 (9.8) | 9 (9.5) | 8 (10.1) | |
| SVG | 1 (0.7) | 0 (0.0) | 1 (1.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Type B2/C lesion | 102 (75.6) | 46 (80.7) | 56 (71.8) | 135 (77.6) | 82 (86.3) | 53 (67.1) | 0.68 |
| Bifurcation lesion | 7 (5.2) | 3 (5.3) | 4 (5.1) | 15 (8.6) | 10 (10.5) | 5 (6.3) | 0.44 |
| Aorta-ostial lesion | 13 (9.6) | 5 (8.8) | 8 (10.3) | 19 (10.9) | 8 (8.4) | 11 (13.9) | 0.71 |
| In-stent restenosis | 11 (8.1) | 5 (8.8) | 6 (7.7) | 13 (7.5) | 7 (7.4) | 6 (7.6) | 0.83 |
| Heavy calcification | 58 (43.0) | 24 (42.1) | 34 (43.6) | 73 (42.0) | 50 (52.6) | 23 (29.1) | 0.76 |
| CTO | 5 (3.7) | 4 (7.0) | 1 (1.3) | 19 (10.9) | 12 (12.6) | 7 (8.9) | 0.025 |
| Pre-PCI FFR guidance | 25 (18.5) | 9 (15.8) | 16 (20.5) | 47 (27.0) | 24 (25.3) | 23 (29.1) | 0.08 |
| Pre-PCI intravascular imaging guidance | 19 (14.1) | 7 (12.3) | 12 (15.4) | 41 (23.6) | 28 (19.5) | 13 (16.5) | 0.14 |
| Predilatation | 88 (65.2) | 36 (63.2) | 52 (66.7) | 124 (71.3) | 68 (71.6) | 56 (70.9) | 0.27 |
| NC balloon | 32 (23.7) | 13 (22.8) | 19 (24.4) | 50 (28.7) | 25 (26.3) | 25 (31.6) | 0.35 |
| Stenting | |||||||
| > 1 stent implanted | 44 (32.6) | 19 (33.3) | 25 (32.1) | 68 (39.1) | 45 (47.4) | 23 (29.1) | 0.24 |
| Stent diameter (mm) | 3.0 (3.0−3.5) | 3.0 (2.8−3.4) | 3.0 (3.0−3.5) | 3.0 (3.0−3.5) | 3.0 (3.0−3.5) | 3.0 (2.9−3.5) | 0.52 |
| Total stent length (mm) | 30.0 (18.0−40.0) | 30.0 (15.0−40.0) | 26.0 (18.0−40.0) | 30.0 (18.0−50.0) | 37.0 (18.0−56.3) | 28.0 (15.0−44.0) | 0.061 |
| Postdilatation | 96 (71.1) | 40 (70.2) | 56 (71.8) | 120 (69.0) | 68 (71.6) | 52 (65.8) | 0.68 |
| NC balloon | 85 (63.0) | 35 (61.4) | 50 (64.1) | 96 (55.2) | 50 (52.6) | 46 (58.2) | 0.17 |
| Post-PCI physiology | 135 (100.0) | 174 (100.0) | NA | ||||
| Pd/Pa distal | 0.93 (0.92−0.96) | 0.94 (0.92−0.97) | 0.93 (0.02−0.96) | 0.94 (0.91−0.96) | 0.94 (0.91−0.96) | 0.94 (0.92−0.96) | 0.97 |
| FFR distal | 0.85 (0.81−0.87) | 0.85 (0.80−0.87) | 0.86 (0.81−0.88) | 0.85 (0.81−0.87) | 0.84 (0.80−0.86) | 0.85 (0.82−0.87) | 0.55 |
| FFR ≤ 0.80 | 31 (23.0) | 16 (28.1) | 15 (19.2) | 40 (23.0) | 24 (25.3) | 16 (20.3) | 0.95 |
| FFR gradient over stent | 0.04 (0.02−0.07) | 0.04 (0.01−0.07) | 0.04 (0.02−0.06) | 0.04 (0.02−0.06) | 0.05 (0.02−0.07) | 0.04 (0.02−0.05) | 0.45 |
| B. Patient-level analysis | NSTE-ACS (n = 130) | CCS (n = 161) | p** | ||||
|---|---|---|---|---|---|---|---|
| All (n = 130) | IVUS (n = 56) | Control (n = 74) | All (n = 161) | IVUS (n = 89) | Control (n = 72) | ||
| Procedural time (min) | 88.5 (70.8−110.3) | 91.5 (74.0−119.8) | 83.0 (62.5−107.3) | 83.0 (65.5−108.0) | 83.0 (69.0−10.3) | 84.5 (59.3−121.5) | 0.17 |
| Contrast use (mL) | 130.0 (100.0−180.0) | 140.0 (102.5−183.8) | 120.0 (90.0−157.5) | 120.0 (95.0−160.0) | 120.0 (90.0−157.5) | 120.0 (100.0−170.0) | 0.19 |
| Fluoroscopy time (min) | 16.7 (12.2−24.1) | 17.4 (11.1−23.5) | 16.1 (9.0−24.7) | 17.3 (10.5−24.5) | 15.9 (11.1−23.5) | 17.7 (9.0−24.7) | 0.58 |
- Note: Values are presented as median (25th−75th percentiles) or n (%).
- Abbreviations: CCS = chronic coronary syndrome, CTO = chronic total occlusion, FFR = fractional flow reserve, LAD = left anterior descending, LCx = left circumflex, LM = left main, NA = not applicable, NC = non-compliant, NSTE-ACS = non-ST-segment elevation acute coronary syndrome, PCI = percutaneous coronary intervention, RCA = right coronary artery, SVG = saphenous vein graft.
- * The vessel-level analysis (part A of the tale) includes procedural characteristics before randomization.
- ** p value for all NSTE-ACS versus all CCS.
Apart from a lower percentage of patients treated for chronic total occlusion and a lower rate of hypercholesterolemia in the NSTE-ACS group, baseline and procedural characteristics were comparable between both groups. The study vessel was the left anterior descending in most patients (76.3% in the NSTE-ACS group vs. 72.4% in the CCS group).
In both groups, the median post-PCI FFR after angiographically successful PCI was 0.85 (0.81−0.87) (p = 0.55) and post-PCI FFR was ≤ 0.80 in 23.0% (p = 0.95) (Figure 1 and Table 2).
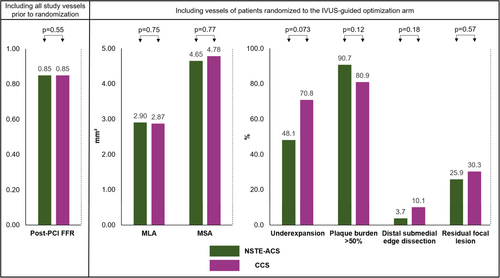
3.2 Pre Optimization IVUS Findings
In patients randomized to the IVUS-guided optimization arm, the NSTE-ACS group involved 57/135 (42.2%) vessels and the CCS group 95/174 (54.6%) vessels (Table 3).
| Vessel-level analysis | NSTE-ACS (n = 57) | CCS (n = 95) | p |
|---|---|---|---|
| Successful post-PCI IVUS | 54/57 (94.7) | 89/95 (93.7) | NA |
| Quantitative parameters | |||
| Minimal lumen area (mm2) | 2.90 (2.02-4.02) | 2.87 (2.33-3.82) | 0.75 |
| Minimal stent area (mm2) | 4.65 (3.61-5.25) | 4.78 (3.54-6.07) | 0.77 |
| Qualitative parameters | |||
| None | 10 (18.5) | 7 (7.9) | 0.14 |
| Underexpansion* | 26 (48.1) | 63 (70.8) | 0.073 |
| Malapposition | 15 (27.8) | 31 (34.8) | 0.38 |
| Edge dissection proximal | 6 (11.1) | 11 (12.4) | 0.82 |
| Submedial | 1 (1.9) | 2 (2.2) | a |
| Edge dissection distal | 7 (13.0) | 15 (16.9) | 0.53 |
| Submedial | 2 (3.7) | 9 (10.1) | 0.18 |
| Edge hematoma | 2 (3.7) | 9 (10.1) | 0.18 |
| Residual focal lesion | 14 (25.9) | 27 (30.3) | 0.57 |
| Proximal | 7 (13.0) | 14 (15.7) | 0.65 |
| Distal | 8 (14.8) | 15 (16.9) | 0.76 |
| Any disease** | 38 (70.4) | 76 (85.4) | 0.15 |
| Optimal stenting criteria met*** | 2 (3.7) | 11 (12.4) | 0.10 |
| Optimal stent expansion | 41 (75.9) | 58 (65.2) | 0.31 |
| Plaque burden in reference area < 50% | 5 (9.3) | 17 (19.1) | 0.12 |
| No submedial edge dissection > 3 mm | 53 (98.1) | 82 (93.2) | 0.22 |
- Note: Values are presented as median (25th−75th percentiles) or n (%).
- Abbreviations: CCS = chronic coronary syndrome, IVUS = intravascular ultrasound, NA = not applicable, NSTE-ACS = non-ST-segment elevation acute coronary syndrome, PCI = percutaneous coronary intervention.
- * According to MUSIC criteria.
- ** Including underexpansion, malapposition, proximal or distal focal lesion or edge hematoma.
- *** According to ULTIMATE criteria.
- a No convergence.
Post-PCI IVUS findings revealed a trend toward less residual disease in vessels of patients with NSTE-ACS as compared to CCS (70.4% vs. 85.4%, p = 0.15). The latter was driven by lower rates of underexpansion (48.1% vs. 70.8%, p = 0.073), malapposition (27.8% vs. 34.8%, p = 0.38), residual focal lesions (25.9% vs. 30.3%, p = 0.57), and edge hematomas (3.7% vs. 10.1%, p = 0.18). Optimal stenting criteria pre optimization were met in 3.7% of vessels in the NSTE-ACS group versus 12.4% of vessels in the CCS group (p = 0.10). The latter was mainly due to the presence of plaque burden > 50% in the reference segments (Figure 1). IVUS-defined post-PCI MLA and MSA were comparable between both groups.
3.3 IVUS-Guided Optimization Strategy
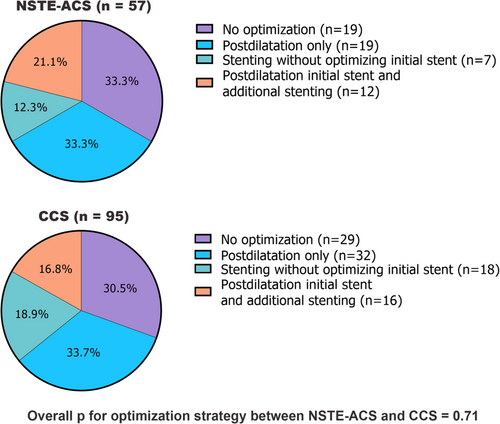
Protocol mandated IVUS-guided optimization was subsequently performed in 38/57 (66.7%) vessels of NSTE-ACS patients and 66/95 (69.5%) vessels of CCS patients. No significant differences in optimization strategy were observed between vessels of patients with NSTE-ACS and CCS (p = 0.71) (Figure 2).
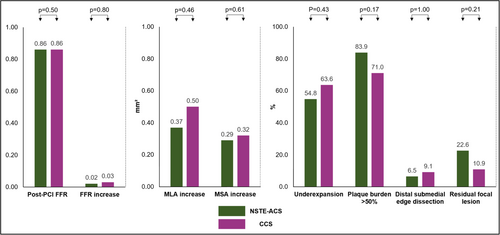
3.4 Final Physiology and IVUS Findings in Optimized Vessels
In optimized vessels of patients randomized to the IVUS-guided optimization arm, FFR increase was significant in both NSTE-ACS (0.02 [0.01−0.05], p = 0.007) and CCS (0.03 [0.00−0.07], p < 0.001), but did not differ significantly between groups (p = 0.80) (Table 4). In addition, 19.4% of the optimized vessels in the NSTE-ACS group reached a final FFR ≥ 0.90 as compared to 20.3% in the CCS group (p = 0.92).
| Vessel-level analysis | NSTE-ACS (n = 38) | CCS (n = 66) | p |
|---|---|---|---|
| Final physiology | 36/38 (94.7) | 64/66 (97.0) | NA |
| Pd/Pa distal | 0.94 (0.92−0.97) | 0.95 (0.92−0.98) | 0.60 |
| FFR distal | 0.86 (0.81−0.89) | 0.86 (0.82−0.89) | 0.50 |
| FFR ≤ 0.80 | 8 (22.2) | 9 (14.1) | 0.30 |
| FFR ≥ 0.90 | 7 (19.4) | 13 (20.3) | 0.92 |
| FFR gradient over stent | 0.03 (0.01−0.04) | 0.03 (0.01−0.05) | 0.85 |
| Median FFR increase | 0.02 (0.01−0.05) | 0.03 (0.00−0.07) | 0.80 |
| Mean FFR increase | 0.033 ± 0.068 | 0.033 ± 0.048 | |
| Successful postoptimization IVUS* | 31/38 (81.6) | 55/66 (83.3) | NA |
| Quantitative parameters | |||
| Increase in MLA (mm2) | 0.37 (0.04-1.14) | 0.50 (0.09-1.51) | 0.46 |
| Increase in MSA (mm2)a | 0.29 (0.13−0.77) | 0.32 (0.17−1.00) | 0.61 |
| Qualitative parameters | |||
| None | 4 (12.9) | 8 (14.5) | 1.00 |
| Underexpansion** | 17 (54.8) | 35 (63.6) | 0.43 |
| Malapposition | 8 (25.8) | 18 (32.7) | 0.50 |
| Edge dissection proximal | 3 (9.7) | 3 (5.5) | 0.66 |
| Submedial | 0 (0.0) | 0 (0.0) | NA |
| Edge dissection distal | 7 (22.6) | 9 (16.4) | 0.48 |
| Submedial | 2 (6.5) | 5 (9.1) | 1.00 |
| Edge hematoma | 2 (6.5) | 4 (7.3) | 1.00 |
| Residual focal lesion | 7 (22.6) | 6 (10.9) | 0.21 |
| Proximal | 3 (9.7) | 1 (1.8) | 0.13 |
| Distal | 4 (12.9) | 5 (9.1) | 0.72 |
| Any disease*** | 25 (80.6) | 44 (80.0) | 0.94 |
| Optimal stent criteria met**** | 4 (12.9) | 13 (23.6) | 0.22 |
| Optimal expansion | 22 (71.0) | 42 (76.4) | 0.58 |
| Plaque burden in reference area < 50% | 5 (16.1) | 16 (29.0) | 0.17 |
| No submedial edge dissection > 3 mm | 30 (96.8) | 53 (96.4) | 1.00 |
- Note: Values are presented as median (25th−75th percentiles), mean ± standard deviation, or n (%).
- Abbreviations: CCS = chronic coronary syndrome, FFR = fractional flow reserve, IVUS = intravascular ultrasound, MLA = minimal lumen area, MSA = minimal stent area, NA = not applicable, NSTE-ACS = non-ST-segment elevation acute coronary syndrome.
- * Including a successful post-PCI and final IVUS.
- ** According to MUSIC criteria.
- *** Including underexpansion, malapposition, proximal or distal focal lesion or edge hematoma.
- **** According to ULTIMATE criteria.
- a In vessels optimized with postdilatation only (scenario 1).
With respect to the final IVUS findings, any residual disease was present in 80.6% of optimized vessels in the NSTE-ACS groups and 80.0% of optimized vessels in the CCS group (p = 0.94). Optimal stenting criteria following optimization were met in 12.9% of the optimized vessels in the NSTE-ACS group versus 23.6% of optimized vessels in the CCS group (p = 0.22).
In optimized vessels a significant increase in MLA was observed in the optimization segment for both NSTE-ACS (0.37 mm2 [0.04−1.14], p < 0.001) and CCS (0.50 mm2 [0.09−1.51], p < 0.001). More specifically, in vessels optimized by postdilatation only (scenario 1), a significant increase in MSA was observed in patients presenting with NSTE-ACS (0.29 mm2 [0.13−0.77], p = 0.004) and CCS (0.32 mm2 [0.17−1.00], p = 0.001). These increases in MLA and MSA did not differ significantly according to clinical presentation with either NSTE-ACS or CCS (Figure 3).
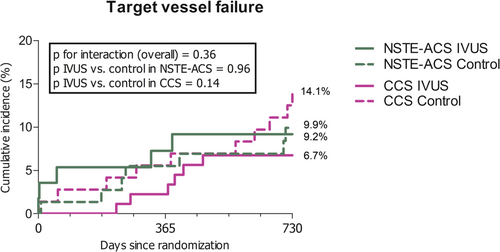
3.5 Clinical Outcome
Clinical follow-up was complete for 96.9% of patients. No significant difference in 2-year TVF and clinically driven TVR was observed in patients randomized to the IVUS-guided optimization arm versus the control arm (TVF: 7.7% vs. 12.0%, HR 0.65, 95% CI 0.30−1.74, p = 0.25, and TVR: 3.6% vs. 9.3%, HR 0.39, 95% CI 0.14−1.08, p = 0.059, respectively) (Supporting Information S1: Table 1). No signs of heterogeneity of the treatment effect based on clinical presentation with NSTE-ACS or CCS were observed for 2-year TVF (p value for interaction = 0.36) (Figure 4).
4 Discussion
The main findings of the present study can be summarized as follows: In patients presenting with NSTE-ACS versus CCS who underwent FFR-directed IVUS-guided optimization (1) post-PCI FFR and pre optimization IVUS findings did not differ significantly, (2) optimization strategy was similar and resulted in comparable improvements in FFR, MLA, and MSA, and (3) the clinical impact of IVUS-guided optimization versus no further treatment showed no signs of heterogeneity between both clinical cohorts.
In the setting of ACS, impaired microvascular function with decreased responsiveness to hyperemic agents has been associated with higher FFR values, potentially leading to underestimation of lesion significance and overestimation of procedural success [14]. To the best of our knowledge, this is the first study assessing the impact of clinical presentation on the efficacy of post-PCI optimization using both FFR and IVUS.
Within the FFR REACT trial, the median post-PCI FFR at the time of randomization was similar in patients presenting with NSTE-ACS and CCS. This finding correlates with previous work observing no physiological difference after stenting between both clinical settings [10, 15]. Furthermore, it strengthens the assumption that the use of post-PCI FFR in NSTE-ACS patients is feasible, with a preserved microvascular function allowing maximum hyperemia [16]. Of note, the latter does not hold for patients presenting with ST-segment elevation myocardial infarction, especially if a higher microvascular resistance is observed [17-19].
Qualitative and quantitative post-PCI IVUS findings did not differ significantly between NSTE-ACS and CCS patients, although numerically lower rates of underexpansion and residual focal lesions were observed in the NSTE-ACS group. The latter could suggest different plaque phenotypes with more dispersed plaque instability in ACS and more extensive disease in CCS [20].
Subsequent PCI optimization was attempted in 68.4% of patients randomized to IVUS-guided optimization and was comparable between patients presenting with NSTE-ACS and CCS. Noteworthy, the optimization rate was higher as compared to studies in which optimization was driven by physiology alone [8-10]. This could be explained by recent work demonstrating that post-PCI IVUS (1) facilitates interpretation of underexpansion, stenting-related complications and identification of residual focal lesions, (2) alters optimization strategy in up to 50% of cases as compared to post-PCI FFR pullback data alone, and (3) has limited agreement with physiological pressure gradients to identify suboptimal PCI results [11, 21, 22]. That said, the optimization strategy in this study did not differ between patients presenting with either NSTE-ACS or CCS, with similar increases in FFR, MLA and MSA being observed for both groups. These findings demonstrate that the use of IVUS to guide PCI optimization in patients with low post-PCI FFR is effective in both patients presenting with NSTE-ACS and CCS.
In patients with a post-PCI FFR < 0.90, we found that optimal stenting criteria (according to the ULTIMATE trial) were met in merely 3.7% of vessels in the NSTE-ACS group versus 12.4% in the CCS group (p = 0.10), figures that only marginally increased following optimization (12.9% vs. 23.6%, respectively [p = 0.22]) [23]. Scrutinizing these results revealed that the low success rates were mainly driven by the inability to reach “disease-free” reference segments with a plaque burden < 50%. Of note, the FFR REACT trial was initiated in October 2017 and did not include plaque burden ≥ 50% in the reference segment(s) as an IVUS-defined target for optimization [13]. The results of our trial should be considered in light of the contemporary understanding of the impact of edge plaque burden on future adverse events [23-25].
Finally, the 2-year clinical outcomes of the FFR REACT trial did not differ significantly between patients with a post-PCI FFR < 0.90 randomized to IVUS-guided optimization versus no further treatment, irrespective of clinical presentation with either NSTE-ACS or CCS. Continuing follow-up will provide more insights in the potential beneficial impact of FFR-directed PCI optimization using HD-IVUS.
4.1 Limitations
The present study was subject to several limitations. First, this was a prespecified analysis of a single-center randomized trial which ultimately appeared to be underpowered. Second, caution is needed for extrapolating these findings to NSTE-ACS and CCS patients with a post-PCI FFR ≥ 0.90. However, previous registry data demonstrated no significant difference in post-PCI FFR for both clinical subsets [15]. Finally, post-PCI physiology measurements were performed using a microcatheter that may generate slightly lower FFR values as compared to pressure wires, particularly in smaller reference vessel segments (mean difference = −0.02) [26].
5 Conclusions
In patients undergoing FFR-directed IVUS-guided optimization, post-PCI FFR, pre optimization IVUS findings and optimization strategy did not differ significantly between patients presenting with either NSTE-ACS or CCS, with comparable improvements in FFR, MLA and MSA in both clinical cohorts. The clinical impact of PCI optimization on 2-year TVF showed no signs of heterogeneity in relation to clinical presentation.
Acknowledgments
The authors would like to thank Jouke Dijkstra for providing dedicated invasive imaging software (QCU-CMS, Leiden University Medical Center, LKEB, Division of Image Processing, version 4.69) to perform IVUS analysis. The Erasmus University Medical Center received institutional research support from ACIST Medical Systems Inc. The funding party was not involved in any of the study-related activities.
Conflicts of Interest
Joost Daemen received institutional grant/research support from Abbott Vascular, ACIST Medical, Boston Scientific, Medtronic, Microport, Pie Medical, and ReCor medical, and consulting and lecture fees from Abbott, Abiomed, ACIST medical, Boston. Scientific, CardiacBooster, Cardialysis BV, Kaminari Medical, Medtronic Pie Medical, PulseCath, ReCor Medical, Sanofi, and Siemens Health Care. Nicolas van Mieghem received institutional research grant support from Abbott Vascular, Biotronik, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, and Medtronic, and consultancy fees from Abbott, Abiomed, Amgen, Anteris, Boston Scientific, Daiichi Sankyo, JenaValve, Medtronic, PulseCath, and Teleflex. Tara Neleman has received institutional grant support from ACIST Medical. The remaining authors report to have no disclosures. The other authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




