The “Cuttering (Cutting-Dottering Balloon) Technique” for treatment of flow-limiting coronary intramural hematoma
Abstract
Background
Coronary artery dissections are caused by a tear in the vessel endothelium, resulting in blood extravasation into the subintimal space, with subsequent intramural hematoma (IMH). One potential technique to deal with this complication is the use of cutting balloons, however, a significant number of cases experienced distal propagation of the hematoma. We describe a novel technique that enhances the possibility of creating intimal tears between the false and true lumen, aiding in hematoma drainage and restoring distal coronary flow.
Methods
We conducted a retrospective analysis of seven consecutive patients who underwent percutaneous coronary intervention complicated by flow-limiting intramural hematomas. All patients were treated using the “Cuttering Technique,” based on the operators’ preference. Procedural success was defined as achieving a distal thrombolysis in myocardial infarction 3 (TIMI 3) flow.
Results
In five out of seven patients treated with “Cuttering Technique” we observed a complete restoration of TIMI 3 flow into the dissected segment.
Conclusions
Our cases show the effectiveness of the “Cuttering Technique” as a viable approach for managing IMHs. This technique enhances the possibility of creating intimal tears between the false and true lumens, aiding in hematoma drainage and restoring distal coronary flow.
1 INTRODUCTION
Coronary artery dissections with subsequent intramural hematoma (IMH) represent a major challenge during percutaneous coronary intervention (PCI). Coronary dissections can be either spontaneous (spontaneous coronary artery dissection [SCAD]) or iatrogenic and are caused by a tear in the vessel endothelium, resulting in blood extravasation into the subintimal space. The false coronary lumen may compress the true lumen, causing restricted coronary blood flow, myocardial ischemia and necrosis. While in case of non-flow limiting dissections/hematomas a conservative management is generally preferred, flow-limiting ones require prompt intervention to restore distal coronary blood flow. However, managing flow-limiting coronary dissections and IMHs may be particularly challenging due to several technical and procedural difficulties. Among these, the main issues include advancing a guidewire into the distal true lumen and dealing with expanding sub-intimal hematomas, which can continue to compress the true lumen proximally and/or distally even after stent implantation.1-3 If this approach is unsuccessful, techniques derived from chronic total occlusion (CTO) interventions can be used. These include subintimal tracking and re-entry,4 limited antegrade subintimal tracking,5 and antegrade fenestration and re-entry.6 Other distal re-entry options include the Stingray system7 and intravascular ultrasound (IVUS)-guided re-entry technique.8 In contrast, dealing with expanding sub-intimal hematomas is crucial to maintain vessel patency and prevent further compression of the true lumen. This can be particularly complex, as the hematoma may expand and continue to exert pressure even after stent placement. As described in several case reports and case series, one potential technique to deal with coronary dissections and IMHs is the use of cutting balloons (CB).9 In the vast majority of cases described in literature, CBs were used to treat SCAD, mostly located in the left anterior descending artery (LAD). The chosen CBs were either smaller than the reference vessel size10, 11 or sized 1:1 with the reference vessel diameter12 and were inflated to low or nominal pressure. Although CB inflation often resulted in the restoration of distal thrombolysis in myocardial infarction 3 (TIMI 3) coronary flow due to the fenestrations created between the false and the true lumen by the cutting blades, a significant number of cases experienced distal propagation of the hematoma. In fact, while low-pressure inflation of the CB is generally chosen to avoid complications such as perforations, it might be insufficient to create adequate fenestrations and true-to-false lumen connections to decompress the IMH. In this paper, we aim to share our experience with a novel application of CBs called the “Cuttering Technique,” (Cutting-Dottering Balloon Technique) to deal with coronary dissections and IMHs. We collected a case series of seven consecutive patients in which the use of this technique allowed us to effectively evacuate IMHs and restore distal coronary blood flow in different clinical scenario.
2 DESCRIPTION OF THE “CUTTERING TECHNIQUE”
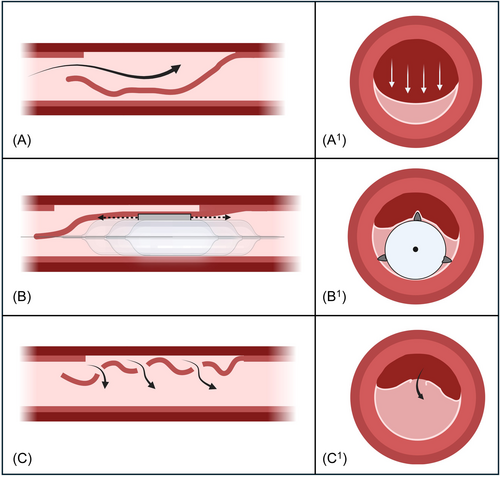
In the “Cuttering Technique,” the CB size should be sized 1:1 with the reference vessel diameter. The CB is then advanced to the area of maximum true lumen compression, where maximum compression of the true lumen and contrast staining can be observed (Central illustration 1A and 1A1). The CB is then inflated to nominal pressure (between 6 and 9 atm) and subsequently gently “dottered” by back-and-forth small pecking movements along the dissected segment (Central illustration 1B and 1B1). These back-and-forth movements bounce the wire up and down, reduce the wire bias, and enhance the chance of successfully CB advancement.13 If the pecking motion is not sufficient to advance and withdraw the CB along the segment compressed by the hematoma, it is possible to partially deflate the CB (ideally between 2 and 4 atm) and continue the “dottering” motion during and after the CB deflation. Multiple CB inflations and deflations might be needed. When the “Cuttering Technique” is successful the result can be seen immediately, with the disappearance of the contrast staining in the affected area, which is a direct sign of the complete drainage of the IMH (Central illustration 1C and 1C1). IVUS investigation can also confirm the effectiveness of this technique as it offers complete vessel visualization in terms of both circumferential and longitudinal hematoma extent. Furthermore, it can provide additional information regarding the blood flow into the analyzed segment: usually, normal intraluminal intensity of blood speckle on IVUS appears as dark gray, while a slow moving or stagnant blood (tight stenosis or hematoma) appears as bright gray blood speckles. After an effective “Cuttering Technique,” IVUS can detect both the creation of multiple fenestrations between the false and the true lumen, and the change in gray intensity of blood speckle, turning from bright (stagnant blood flow) to dark (normal blood flow). These signs correlate with an effective drainage of the hematoma with subsequent distal flow restoration, that can be confirmed with contrast injection.
3 METHODS
3.1 Data collection and patient selection
We conducted a retrospective analysis of seven consecutive patients who underwent PCI complicated by flow-limiting IMHs at Humanitas Research Hospital, Rozzano, Italy and Ospedale Papa Giovanni XXIII, Bergamo, Italy. All patients were treated using the “Cuttering Technique,” based on the operators’ preference. Procedural success was defined as achieving a distal TIMI 3 flow. The study adhered to the ethical principles outlined in the Declaration of Helsinki.
4 CASE EXAMPLES
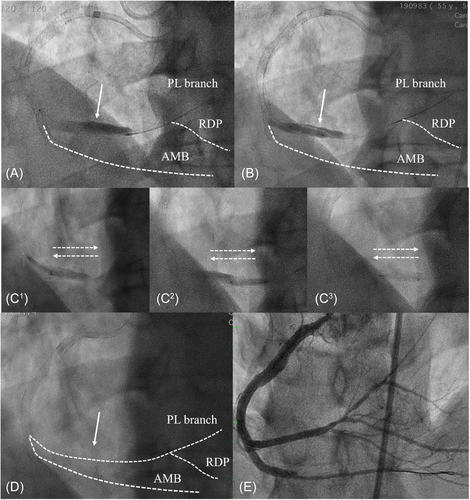
Case 1: A 65-year-old male presented with typical angina over exertion, evidence of inferior wall ischemia at myocardial perfusion imaging (>10%) and right coronary artery (RCA) CTO at angiography. Due to the lifestyle-limiting symptoms and the large size of ischemia, an attempt was made to perform RCA CTO PCI. After careful evaluation, an antegrade approach was opted for as primary recanalization attempt strategy. Nonetheless, the blunt stump and bridging collaterals contributed to an unsuccessful antegrade wire escalation (AWE), and a retrograde approach via epicardial branches into the right posterolateral (PL) branch was then attempted. After successful reverse-cart in the mid RCA an RG3 (Asahi Intecc, Seto, Aichi, Japan) wire was then externalized followed by several antegrade balloon dilatations. Afterwards, an inadvertent antegrade injection was performed propagated a large flow limiting dissection into the PL branch (Figure 1A). To drain the IMH, we inflated a 3.0 mm Wolverine CB (Boston Scientific Corporation) along the entire dissected segment without having a complete drainage of the contrast staining (Figure 1B). We then performed the “Cuttering Technique” with the same 3.0 CB (Figure 1C1–C3), causing an immediate and complete resolution of the IMH that was evidenced by the disappearance of the contrast staining (Figure 1D; File S1). The procedure was completed with multiple stents implantation achieving a final TIMI 3 flow in all the branches (Figure 1E).
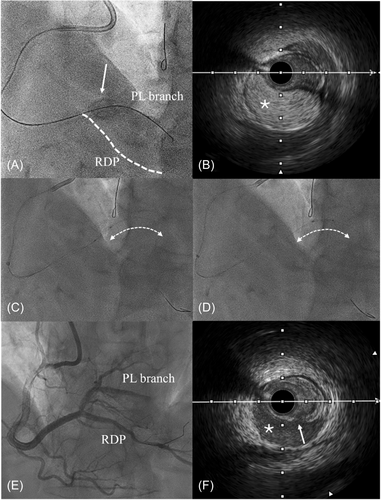
Case 2: A 66-year-old male with history of previous LAD and left main PCI, presented with typical angina over exertion, large inferior wall ischemia at stress cardiac magnetic resonance (>10%) and RCA CTO at angiography. RCA CTO PCI was planned and a successful AWE was performed. After successful microcatheter advancement into the distal true lumen, the CTO guidewire was withdrawn and a workhorse one was advanced. During balloon inflation, due to the severe calcification, we had an unintentional balloon rupture that created a large dissection involving the ostium of the PL branch (Figure 2A). Any further contrast media injections were avoided and an IVUS evaluation showed a huge IMH (bright gray blood speckles; Figure 2B) compressing the true lumen. Due to these finding we performed the “Cuttering Technique” with a 1:1 size Wolverine CB. After several CB inflations to nominal pressure and subsequent reiterated “back-and-forth movements along the dissected segments (Figure 2C,D), we obtained a complete drainage of the IMH. IVUS showed the presence of several intimal tears (Figure 2F) between the false and the true lumen, favoring the drainage of the IMH (dark gray blood speckles) with subsequent distal flow restoration. The procedure was completed with multiple stents implantation in the proximal and mid RCA, without any further stent implantation into both the posterior descending artery and the PL branches (Figure 2E).
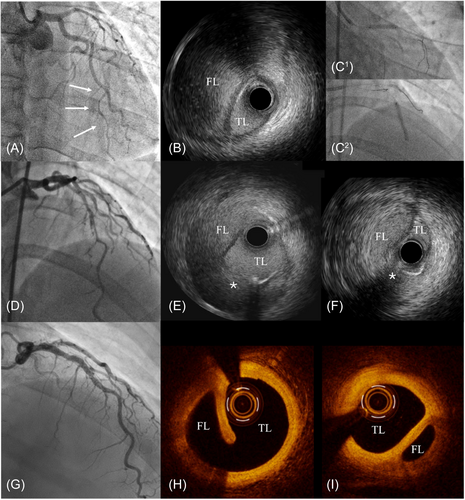
Case 3: A 45-year-old female with new onset chest pain and anterior ST elevation myiocardial infarction was admitted to our hospital. The coronary angiography showed a SCAD of the mid LAD with distal TIMI 1 flow. After careful wiring of the dissected segment with a Suoh 0.3 wire (Asahi Intecc, Seto, Aichi, Japan) (Figure 3A), we performed IVUS that confirmed the intraluminal position of the wire and a long dissection with a large IMH compressing the true lumen (Figure 3B). Due to this finding, we performed the “Cuttering Technique” with a 1:1 size Wolverine CB that was dottered several times along the dissected segment (Figure 3C1 and C2), allowing the restoration of a distal TIMI 3 flow (Figure 3D). A final IVUS evaluation showed the new-generated intimal tears between the false and the true lumen, with significant reduction of the dimension of the IMH (Figure 3E,F). According to the best practice in the treatment of SCAD,14 no stent was implanted. A 6-month angiography with optimal coherence tomography imaging showed LAD patency (Figure 3G) with complete reabsorption of the IMH and partial heal of the intimal tears created by the “Cuttering Technique” (Figure 3H,I).
A summary description of the clinical cases in which we adopted the “Cuttering Technique” to deal with IMHs are reported in Table 1.
| Involved artery | Clinical presentation | Mechanism of the dissection/IMH | Final TIMI flow | |
|---|---|---|---|---|
| Case 1 | RCA CTO | Stable angina | Reverse CART | 3 |
| Case 2 | RCA CTO | Stable angina | Balloon rupture | 3 |
| Case 3 | LAD | STEMI | SCAD | 3 |
| Case 4 | RCx CTO | Stable angina | ADR | 2 |
| Case 5 | RCA | Stable angina | Guiding catheter-induced | 2 |
| Case 6 | LAD | Stable angina | Balloon predilatation | 3 |
| Case 7 | OM | Silent ischemia | Stent delivery related | 3 |
- Abbreviations: ADR, antegrade dissection and reentry; CART, controlled antegrade and retrograde subintimal tracking; CTO, chronic total occlusion; IMH, intramural hematoma; LAD, left anterior descending artery; OM, obtuse marginal; RCA, right coronary artery; RCx, ramus circumflex artery; SCAD, spontaneous coronary artery dissection; STEMI, ST-elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
5 DISCUSSION
- ▪
Concentrated force: the sliding motion causes the blade to cut through the material at a narrower point at a time, rather than along the entire length of the cut simultaneously. This concentrates the force on a smaller surface area, increasing the pressure (force/area) at that point, according to Pascal's principle.
- ▪
Progressive cutting: instead of cutting all the material at once, the blade cuts progressively along its edge. This means each part of the material is cut in sequence, requiring less force at any given moment.
Additionally, when the blade slides through the material, it generates microfractures and small localized breaks. These fractures make it easier to continue cutting, as the material is already partially weakened. The sliding motion can also exploit the phenomenon of “tissue fatigue,” where the tissue (i.e., intima) becomes progressively weaker with the repeated application of cyclic stress. Finally, the sliding motion reduces friction compared to constant vertical pressure because the point of contact between the blade and the material continuously changes, reducing heat buildup and minimizing adhesion. In summary, the sliding motion applied during the “Cuttering Technique” allows the force to be concentrated in a smaller area, enables progressive cutting, exploits microfractures and material fatigue, and reduces friction. All these factors make the cutting process more efficient and require less force compared to simple vertical pressure. This is why we are strongly convinced about the effectiveness of this technique in cases where a severe IMH affects the distal coronary blood flow. In such scenarios it is well known that standard CB inflation may result in the restoration of distal TIMI 3 coronary flow, however a significant number of cases still experience distal propagation of the IMH (squeezing phenomenon). The “Cuttering Technique” can increase the chance to create a larger or even higher number of fenestrations between the false and the true lumen, facilitating the drainage of the IMH with subsequent distal flow restoration. In our case series of flow-limiting IMHs, the “Cuttering Technique” was effective to achieve a distal TIMI three flow in five out of seven patients, while in the two remaining cases it allowed us to obtain a final TIMI 2 flow, without any clinical sequelae. On the flip side, the main drawback of this technique is that it may not be recommended in certain situations, such as when there are excessive calcifications, extreme vessel tortuosity or in case of guidewire extraluminal tracking. Indeed, excessive calcifications may reduce the “Cuttering Technique” effectiveness, potentially increasing the risk of coronary perforation. Likewise, severe vessel tortuosity can hinder the CB control during “dottering” and increase the risk of creating vessel damage. Last but not least, in case extraluminal tracking, inflating a CB into the subintimal space is not recommended due to the risk of major perforations.
6 CONCLUSION
The current lack of established guidelines for treating IMHs has led to the widespread use of a CB at the hematoma site to restore distal blood flow. Even though CB inflation is often sufficient, it may not always be enough or could lead to distal propagation of the hematoma. Our cases show the effectiveness of the “Cuttering Technique” as a viable approach for managing IMHs. This technique enhances the possibility of creating intimal tears between the false and true lumens, aiding in hematoma drainage and restoring distal coronary flow.
ACKNOWLEDGMENTS
Open access funding provided by BIBLIOSAN.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.