Mobile bedside ductus arteriosus closure in severely premature neonates using only echocardiographic guidance
Abstract
Background
Transcatheter closure of the patent ductus arteriosus (PDA) in premature infants is currently dependent on fluoroscopic guidance and transportation to the catheterization laboratory.
Aim
We describe a new echocardiographically guided technique to allow our team to move to the bedside at the neonatal intensive care unit (NICU) of the referring center for percutaneous treatment of PDA in premature infants.
Methods
This is a single-center, retrospective, primarily descriptive analysis. Clinical details about the procedure, its outcomes, and complications were collected.
Results
Fifty-eight neonates with a median weight of 1110 g (range 730–2800) and postnatal age of 28 days (range 9–95) underwent percutaneous PDA closure. Five of them were treated in our center with ultrasound guidance only and the other 53 in 18 different neonatology units in 12 towns. The median duration of the procedure was 40 min (range 20–195 min). There were no procedural deaths. There was one residual shunt for 3 weeks, in all other patients the duct closed completely in the first few hours after the intervention. In one patient the procedure had to be interrupted because of a pericardial effusion which had to be drained, the PDA was closed successfully interventionally 5 days later. One device-related aortic coarctation had to be stented. One embolization and one late migration occurred and required treatment.
Conclusions
Echocardiographically guided transcatheter closure of the PDA in prematures was repeatedly possible and allowed that the procedure is performed at the bedside at the NICU with an acceptable rate of complications.
1 INTRODUCTION
In spite of the high incidence of patent ductus arteriosus (PDA) in premature infants and its potentially negative effects on the clinical course of these fragile patients, uncertainty exists about optimal management.1, 2 Traditionally, premature patients with PDA have been treated pharmacologically, surgically or with a conservative observational approach. All of these methods have their drawbacks or in some patients are even contraindicated. Following the first report of transcatheter PDA closure (TCPC) in a premature infant in 2001,3 the early experience remained limited to highly selected patients and small cases series.4-8 Recently, with the approval of the Piccolo device (Abbott Medical) for this specific indication,9 the procedure has become more available to an number of premature babies. While TCPC in preterm infants has been shown to be safe and effective, currently, the procedure is typically performed in the catheterization suite. This requires the use of ionizing radiation, contrast agents in some centers, and most importantly, transport out of the neonatal intensive care unit (NICU). In many cases, this involves transport to a tertiary care center medical center. We developed a new method for performing TCPC in preterm infants using only echocardiographic guidance allowing these fragile patients to be treated in an incubator within their original NICU.10 Here, we report our experience with the first 52 patients and provide technical details about the procedure.
2 METHODS
The study includes all premature patients who were treated with TCPC using ultrasound guidance only. According to the local legal regulations, the procedure was performed after informed consent from the parents with detailed information about risks and benefits of this novel treatment. According to the local regulations, the parents gave their informed consent for anonymous scientific evaluation of the data.
2.1 Patient selection
The indications for PDA closure were primary determined by the attending neonatologists from the referring neonatology units. Typically, patients had a failed trial of pharmacological closure of the PDA and were considered for this procedure only if they had a structurally normal heart.
2.2 Procedural development
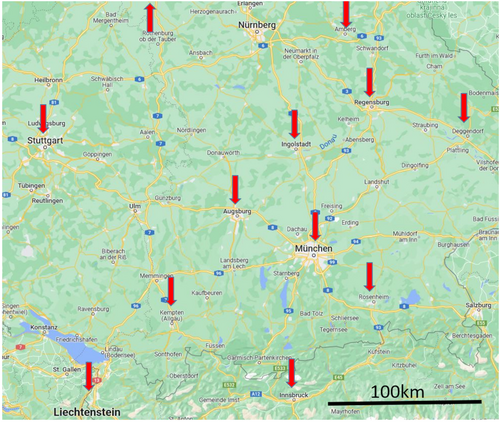
All procedures were guided solely by echocardiography without the use of any fluoroscopy. The first 3 cases were performed in the catheterization suite (case #1 on the catheterization table and cases #2 and #3 in an incubator within the lab), so as to have fluoroscopic backup if needed. Cases #4 and #5 were performed in an incubator in the pediatric cardiology intensive care unit within our own institution. All the other procedures were performed in the NICUs of 18 referring clinics in 12 towns surrounding Munich, Germany. In the first of these cases, hospitals with available adult cardiology catheterization laboratories as backup were chosen.
2.3 Procedural details
Following the initial five cases, all remaining cases were performed in the referring NICU and the patients were not moved from their incubators (Figure 1). Patients were sedated intravenously by the attending neonatologists. Typically, respiratory support was intensified from baseline for the procedure based on the judgment of the neonatologist. For example, patients on high flow cannula were placed on continuous positive pressure ventilation (CPAP) and patients receiving CPAP were intubated and mechanically ventilated. This resulted in a good tolerance of the procedure. Immediately before the intervention transthoracic echocardiography was performed by our interventional team with focus on the morphology and size of the PDA. Based upon these measurements, a Piccolo device size was determined and the device was prepared in the usual fashion before gaining vascular access. A device with a diameter at least 1 mm larger than the narrowest part of the ductus was chosen. Typically, a short device of 2 mm was selected; only in large, long PDAs a 4 mm long device was used. Before vessel puncture, all catheters and guidewires were prepared. A 4Fr hydrophilic catheter (Bentson-Hanafee-Wilson Glidecath, Terumo Europe) was modified by cutting the proximal end for a total catheter length of 30 cm. The cut end of the catheter was sealed with the flushing port of an IVA 3Fr sheath (Balt Extrusion). This catheter was used in a combination with either a 50 cm long 0.035″ Radiofocus guidewire (Terumo Europe) or with a shortened Progreat microcatheter (Terumo Europe) in combination with a coronary 0.014″ Balanced Middleweight Universal guidewire (Abbott Vascular) which was shortened as well by cutting it with sterile scissors (Figure 2). The second combination was used only in our first cases.
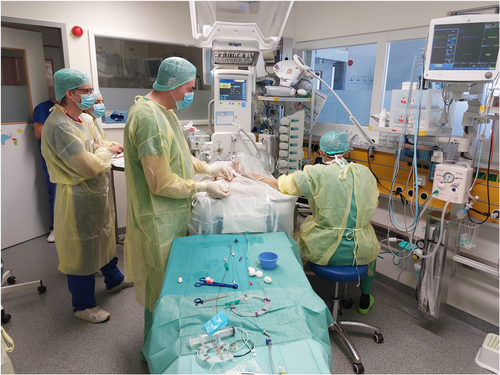

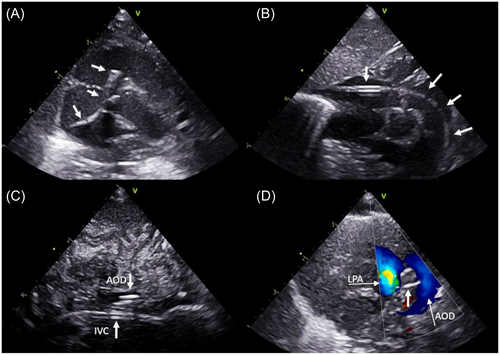
The femoral vein was punctured under ultrasound guidance and a standard 4Fr vascular sheath was inserted. A bolus of 30 units/kg heparin was administered if not contraindicated and a prophylactic single shot cefuroxime was given. All catheter and guidewire manipulations were guided solely by continuous transthoracic echocardiography using standardized views (Figure 3). Using a long-axis subcostal view with angulation toward the inferior caval vein, a standard 0.035″ guidewire was introduced into the right atrium, and over it, the shortened hydrophilic catheter was advanced until its tip was clearly identified echocardiographically. Slight rotation of the transducer showed the junction of the inferior caval vein to the right atrium and the tricuspid valve and was optimal for directing the catheter toward the tricuspid valve and carefully advancing it into the right ventricle. After the catheter was positioned directly behind the tricuspid valve, the transducer position was changed to a standard precordial short-axis view of the right ventricle. The tip of the catheter was orientated with a careful clockwise rotation toward the pulmonary valve and was advanced through it. The guidewire was introduced 1 cm out of the catheter and the whole system then advanced further which typically resulted in the catheter-guidewire system passing through the ductus arteriosus into the descending aorta. A high parasternal short-axis “ductus view” or a short-axis subcostal view was used for visualizing the catheter crossing the PDA. Its position in the descending aorta was additionally confirmed in a subcostal long-axis view of the descending aorta. An atypical long-axis view from the side of the thorax showing both the inferior caval vein and the descending aorta with catheters inside the vessels was useful. In the rare occasion that the system could not be visualized in the descending aorta, the assumption was made the catheter had entered a branch pulmonary artery. It was then slowly withdrawn until the tip could be visualized in the main pulmonary artery (MPA) once again and further attempts to cross the PDA were performed using the same technique. Once the catheter tip was visualized at the level of the diaphragm it was exchanged over a 150 cm long 0.035″ Fixed Core guidewire (Cook Medical) for the Amplatzer Torque Vue delivery catheter (Abbott Medical). A subcostal short-axis view of the right ventricle showing the entire part of the guidewire passing through the right heart was used for exchanging and controlling the tension on the system. The selected Piccolo device was advanced to the tip of the delivery catheter and the delivery catheter was slowly withdrawn until the tip was visualized in the upper thoracic descending aorta just opposite the ductus using the high parasternal “ductal view.” With one or two parts of the device outside of the delivery catheter, it was pulled back into the ductus and then rest was developed as well. As previously described,10 we used a combination of two-dimensional, Doppler and color Doppler imaging to examine for residual shunt, aortic or left pulmonary artery obstruction, and device stability. If any of these were suspected the device was retracted back into the delivery catheter and repositioned or replaced before release. Once satisfied the device was in the correct position, it was unscrewed carefully from the delivery cable and the delivery catheter removed. Before removing the vascular sheath from the femoral vein, a postprocedure complete echocardiogram was performed to confirm the final position of the Piccolo device and to rule out any procedural complications such as device migration/embolization, vascular obstruction, or injury of the tricuspid valve. A repeat echocardiogram was recommended to be performed in the evening of the procedure, on the following day, 1 week following the procedure and before discharge. This was done by the responsible pediatric cardiologist for the NICUs.
2.4 Study data and statistical analysis
This is primarily a descriptive study of a new technique. Patient demographics and clinical parameters are presented as median values with ranges.
3 RESULTS
3.1 Patients
Between July 2019 and November 2023, 58 consecutive premature patients with PDA were treated with this technique. Patient demographics are presented in Table 1.
| Weight at birth (g) | 760 (340–2560) |
| Weight at intervention (g) | 1100 (730–2800) |
| Weight < 1000 g N (%) | 24 (41%) |
| Age at intervention (days) | 26 (9-95) |
| History of infection, N (%) | 27 (47%) |
| History of NEC | 8 (14%) |
| Trial of NSAIDs | 56 (97%) |
| Respiratory support before intervention | |
| Oxygen supplementation | 1 (2%) |
| High flow cannula | 7 (12%) |
| CPAP | 16 (28%) |
| Ventilation | 28 (48%) |
| HFOV | 12 (21%) |
| Respiratory support during intervention | |
| High flow cannula | 2 (3%) |
| CPAP | 6 (10%) |
| Ventilation | 43 (74%) |
| HFOV | 7 (12%) |
- Abbreviations: CPAP, continuous positive airway pressure; HFOV, high-frequency oscillatory ventilation; NEC, necrotizing enterocolitis; NSAIDs, nonsteroidal anti-inflammatory drug.
3.2 Interventional results
3.2.1 Interventional details
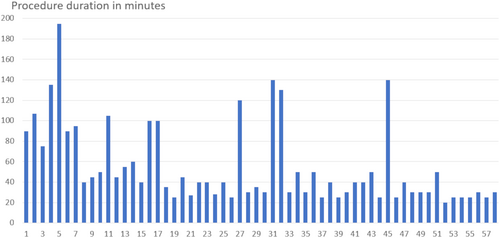
There were no cases in which an attempted procedure was not performed because of bad echocardiographic windows or for some other reason. All procedures were guided only by echocardiography. The first patient was treated on the catheterization table only with ultrasound guidance and without the use of fluoroscopy. The next two patients were managed in the incubator in the catheterization lab and patients #4 and #5—in the incubator on the intensive care unit in our pediatric cardiology center. All the other procedures were performed in the NICUs of 18 referring clinics in 12 towns in the wide surroundings of Munich (Figure 4). The median duration of the procedure (as defined by sheath insertion to sheath removal) was 40 min (range 20–195 min) with a clear tendency toward shorter procedure times with increasing experience (Figure 5). In two patients (weight 750 and 850 g), the ductus was closed from a retrograde approach because of unintentional cannulation of the femoral artery. Both patients received prophylactic heparin infusion for 24 h and had a good peripheral pulse on the punctured leg afterward. In all other patients, the ductus was closed using an antegrade approach from the femoral vein.
3.2.2 Outcomes and complications
There were no procedural deaths. In one 800 g patient after implantation of a 5/2 Piccolo device into a large ductus, residual shunt persisted for 3 weeks before closing completely. No signs of hemolysis were observed. In all other neonates, complete PDA closure was documented within 24 h after the procedure. There was no new tricuspid regurgitation in any of the patients. Four major complications with certain association to the procedure were observed—perforation, embolization, device-related aortic coarctation, and late migration of the device with complete closure of the right pulmonary artery.
In one patient, the procedure resulted in an acute pericardial effusion. This severely sick 1000 g infant was on high-frequency oscilation ventilation with poor echocardiographic windows. In spite of that and because of the critical condition of the patient, for which it was thought the PDA played a significant role, it was decided to proceed with the procedure. During efforts to reach the right ventricular outflow tract with the catheter, an effusion was noted on echocardiography and 8 mL blood were drained percutaneously. Although the patient remained hemodynamically stable it was decided to terminate the procedure. Five days later, the PDA was closed percutaneously using echocardiographic guidance on the NICU.
There was one embolization of a 5/2 Piccolo device in a 900 g patient. This was most probably due to a mishandling or a technical problem with the device. After an uneventful procedure so far, it was noted that the device, 2/3 of which was sticking out of the delivery catheter in the descending aorta, was disconnected from the delivery cable. Although it was possible to pull back the system and position the device properly into the duct, it embolized into the MPA while pushing it out with a wire. The patient remained completely stable, was heparinized, and transported with a helicopter immediately to our center. Three hours after the embolization, the device was retrieved and the duct was then closed uneventfully with a 5/2 device.
One patient, weighting 850 g at the procedure, developed aortic coarctation which required treatment. The patient had an atypical short ductus. During the PDA closure procedure, a Piccolo 4/2 had to be repositioned repeatedly, so that it did not cause obstruction either to the left pulmonary artery or the aortic isthmus. Over the ensuing 2 weeks, the baby developed signs of coarctation of the aorta both clinically and echocardiographically. Using a carotid approach, the aortic isthmus was stented with a coronary stent which led to the resolution of the coarctation. Further reinterventions to enlarge the stent will be required in the future.
There was one migration of the device resulting in complete closure of the right pulmonary artery 2.5 months after the procedure. A large duct was closed with a 5/2 Piccolo in an 1100 g patient. During the procedure and on the next day, a proper position of the occluder was documented. More than 2 months later, the patient weighting 2400 g at that time started deteriorating respiratory, and complete obstruction of the right pulmonary artery by the dislocated Piccolo was diagnosed. The duct was closed. After an unsuccessful attempt to retrieve the device percutaneously, surgical removal of the device was performed. The child recovered uneventfully with good flow into the right pulmonary artery.
One patient (850 g) developed necrotizing enterocolitis (NEC) 1 week after the procedure from which he fully recovered. This patient developed accelerated Doppler flow velocity as high as 2.5 m/s in the descending aorta at the level of the diaphragm following the intervention. It is possible that this resulted from aortic wall damage related to catheter and wire manipulation and had an impact on the development of NEC. Descending aorta flow velocities normalized 3 weeks following intervention.
There were two possible thromboembolic complications related to the procedure. In one patient (1440 g), the contralateral femoral pulse was weakened after the intervention and recovered after 24 h of heparin infusion. In another (1450 g), surgery for testicular torsion was required 36 h after the procedure and the surgeon described a possible macroscopic embolic lesion of the testicle.
4 DISCUSSION
This study shows that interventional closure of PDA in premature infants with echocardiographic guidance only in the NICU of the referring institution without the need of patient transportation is possible with significant team experience. With the presented method we performed the procedure in a series of 58 consecutive patients. The intervention was associated with some complications, which have to be taken into consideration and further refinement of the technique should help minimize their incidence.
In recent years, following Food and Drug Administration approval of the Abbott Piccolo device for PDA closure in infants as small as 700 g,9 TCPC has emerged as a viable treatment option for premature infants with hemodynamically significant PDA.11 Universally, this procedure is being performed in the cardiac catheterization laboratory using a combination of fluoroscopic and echocardiographic guidance and has been shown in experienced hands, to be safe and effective with 6-month success rates > 95% and major complication rates <3%.9 However, the current method has several major drawbacks. First, patients have to be transported to a pediatric cardiology catheterization laboratory which in many cases means transportation to another hospital or at the very least from a NICU to a catheterization suite. Inherent risks during transportation are cardiorespiratory instability, thermodysregulation, accidental endotracheal tube dislodgement, displacement of invasive lines, infusion pump dysfunction leading to discontinuation or purge of inotropes, and incubator malfunction.12 While these risks may be mitigated by the formation of a well-designed premature PDA program, this is not possible in all centers and regions. Additionally, when performed in the catheterization suite, these procedures involve the exposure to ionizing radiation, the long-term effects of which on these immature babies are currently unknown. Finally, when these fragile infants, who often have significant and severe comorbidities are transported to the catheterization laboratory, they are temporality removed from their highly specialized care team in the NICU. For all of these same reasons, surgical PDA ligation in premature infants has moved away from the operational theater and is now routinely performed to the bedside in many centers.13
Based on the abovementioned concerns and noting the successful bedside surgical ligation experience, our group sought to develop a technique for transcatheter premature PDA closure which would accomplish the same goals.
There are some technical issues with our method which have to be discussed. Most importantly, it involves learning to manipulate the catheters and guidewires without X-ray and under ultrasound guidance only. When starting our program, it proved to be useful for the operators to be confident with performing other catheter interventions like Rashkind procedures, atrial septal defects and persistent foramen ovale closures, persistent foramen ovale stenting and others, only with echocardiographic guidance. Providing sufficiently good echocardiographic images during the whole procedure is crucial but difficult. As recently published,14 a detailed echocardiographic imaging of the duct in premature infants is possible, comparable to the angiography and sufficient to decide on the appropriate size of the device to be used. In our experience, the echocardiographic imaging for the procedure is always performed by one of the interventionists who is confident with technical aspects of the procedure. This ensures that the imager is always aware of the next step of the intervention, that he knows exactly what needs to be shown echocardiographically, and secures good cooperation between the imaging and catheterizing physician. Shortening the catheters and wires made their controlled manipulation in the tiny patients in the limited space of the incubator significantly easier. It was useful for us to start with the echocardiographic-guided PDA closures in the catheterization laboratory in our center and move stepwise away to other hospitals when gaining confidence with the procedure. Using structured training, the abovementioned issues and difficulties are resolvable and allow to provide the optimal interventional treatment at the bedside in the neonatology clinic.
The procedure was associated with some complications which are reported with the fluoroscopy-guided intervention as well. Most of them were probably related to the learning curve with the new procedure and should be analyzed carefully to avoid them. Although treatment was possible in all cases, one should be aware, that procedural complications are potentially more difficult to manage in NICU in a peripheral hospital without a special pediatric catheterization laboratory. One possible bailout strategy until getting confident with the intervention is to choose NICUs in hospitals where an adult catherization laboratory is available for the first cases. The most concerning for us was the perforation leading to a significant pericardial effusion. As the patient remained stable and the bleeding did not persist, we presume that the right atrial appendage was perforated. In our opinion, it was the only complication in this series which was directly related to the echocardiographic guidance of the procedure. This patient had poor echocardiographic views because of the intensive high-frequency oscillation ventilation which led to extreme difficulties to perform the procedure in a controllable fashion. We have treated 7 patients on high-frequency oscillation ventilation and these were subjectively the most difficult procedures. In most of them the intensity of the ventilation could be reduced temporary to allow acceptable echocardiography quality. If this is not possible, postponing the intervention, fluoroscopy-guided TCPC, or surgical PDA closure should be considered. Embolizing a device in a distant clinic which does not have a pediatric catheterization laboratory is probably the most feared complication when considering doing the procedure at the bedside in the NICU. In our case the cause of the embolization was not associated with the use of echocardiographic guidance only. The patient remained stable and could be transported to our center for further treatment. Aortic isthmus obstruction is a known complication after PDA closure in premature infants. Cases like ours, in which a short duct is diagnosed and a complete intraductal implantation of even a 2 mm long Piccolo device is not possible, should be probably considered for another treatment method. Migration of the device is described and underlines the need of further echocardiographic follow up after interventional duct closure in premature infants. Because of the tiny dimensions, inadvertent puncture of the femoral artery is possible even when using ultrasound-guided puncture. We strongly advise controlling the position of the guidewire in the right atrium with echocardiography before inserting the sheath. This is easily achievable and became standard in our experience. Even though, PDA closure using an arterial access is possible and technically easier, the risk for injury of the artery is too high. There were two possible thrombotic complications in our series. Cerebral embolic events are extremely improbable with this technique as the catheters are manipulated in the arterial system distally to the cerebral vessels. We routinely administer low dose heparin, regularly flush the catheters between wire exchanges and aim to keep the procedure as short as possible.
The idea of performing bedside percutaneous PDA closure in prematures under echocardiographic guidance is not new to the congenital cardiology interventional community. More than 10 years ago, Bentham et al.15 reported a series of three prematures in whom the ductus was closed interventionally on the NICU with ultrasound guidance only. The authors achieved excellent results in this small group of patients. However, not having the optimal device at that time, they did not use standardized technique and intervened using a femoral artery approach. Using alternative devices other that the Piccolo with bedside TCPC is highly depended on their good visualization. If other devices can be used for that remains unclear, but we suspect that for instance the Microvascular plug (Medtronic plc) would be unsuitable because of the limited imaging possibilities with echocardiography. More recently, Zahn et al.16 published a series of 24 premature patients, in some of whom the PDA closure was performed on the NICU. Similar method is used by the group from Memphis, Tennesee.17 This approach resulted in excellent results, involved, however, a high-quality low radiation mini C-Arm suitable for guidance of the procedure in such tiny patients and a special procedural neonatology bed, both of which are not available in most NICUs. Developing and standardizing our technique, we treated patients on site at the referring NICUs without needing any special equipment. During the first 4 years of the project we worked in 18 clinics providing transportation-free bedside interventional PDA closure in prematures in a large area surrounding Munich, Germany (Figure 4).
Our study has some important limitations. It reports early results of a novel method of treatment in a small group of patients. All procedures were performed by the same interventional team experienced in performing complex procedures in neonates with echocardiographic guidance. There were some important complications which were probably partially related to the learning process with the procedure. Long term outcomes of the procedure especially in comparison to other methods of treatment of the PDA in preterm infants are not known.
5 CONCLUSIONS
Ultrasound-guided percutaneous PDA closure in premature infants was repeatedly feasible in 58 patients and allowed bedside treatment on the NICU. Further experience and improvements of the method should make it a low-risk procedure which can be routinely performed in the incubator on the NICU from many experienced interventional teams. This may become an important tool for improving the outcomes of many premature infants. Studies about the feasibility and risks of complications in larger groups of patients and following controlled studies should help define the indications of this novel treatment.
ACKNOWLEDGEMENTS
Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
Dr. Evan Zahn serves as a proctor for Abbott for the Piccolo device. The remaining authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.