Experience with the ACURATE neo and neo2 transcatheter aortic valves in Spain. The PRECISA (PRospective Evaluation Complementing Investigation with ACURATE devices) registry
Clinical trials identification number: NCT03846557
Abstract
Background
Previous studies have documented a high rate of implantation success with the ACURATE neo2 valve, as well as a reduction in paravalvular leak (PVL) compared to its predecessor, the ACURATE neo. However, there are no studies that have reviewed and compared the long-term clinical and hemodynamic outcomes of these patients.
Aims
This study aimed to evaluate the results of the ACURATE neo transcatheter aortic valve in a real-world context, and to compare the results of the outcomes of both generations of this device (ACURATE neo and ACURATE neo2), with a specific focus on procedural success, safety, and long-term effectiveness.
Methods
A prospective study including all consecutive patients treated with the ACURATE neo device in seven hospitals was conducted (Clinical Trials Identification Number: NCT03846557). The primary endpoint consisted of a composite of adverse events, including mortality, aortic insufficiency, and other procedural complications. As the second-generation device (ACURATE neo2) replaced the ACURATE neo during the study period, hemodynamic and clinical results before admission, at 30 days, and at 1 year of follow-up were compared between the two generations.
Results
A total of 296 patients underwent transcatheter aortic valve implantation with the ACURATE device, with 178 patients receiving the ACURATE neo and 118 patients receiving the ACURATE neo2. In the overall population, the absence of device success occurred in 14.5%. The primary reason for the absence of device success was the presence of para-valvular regurgitation ≥ 2. There were no instances of coronary occlusions, valve embolization, annulus rupture, or procedural deaths. ACURATE neo2 was associated with a significantly higher device success rate (91.7% vs. 82%, p = 0.04), primarily due to a significantly lower rate of para-valvular regurgitation, which remained significant at 1 year.
Conclusion
The use of ACURATE neo and neo2 transcatheter aortic valves is associated with satisfactory clinical results and an extremely low rate of complications. The ACURATE neo2 enables a significantly higher device success rate, primarily attributed to a significant reduction in the rate of PVL.
1 INTRODUCTION
Transcatheter aortic valve implantation (TAVI) has firmly established itself as the foremost therapeutic choice for an expanding cohort of patients afflicted with symptomatic aortic stenosis (AS).1
The inaugural release of the self-expanding ACURATE neo device (Boston Scientific) marked the introduction of self-expanding transcatheter heart valves, bringing a range of benefits, including reduced post-procedural gradients, a lower likelihood of permanent pacemaker dependency and a decreased risk of coronary occlusion during implantation.2, 3 Nevertheless, the incidence of moderate to severe paravalvular leak emerged as its principal drawback, a phenomenon substantiated by two recent randomized clinical trials comparing this device against a balloon-expandable variant (SCOPE I)4 and another self-expanding device (SCOPE II).5 To overcome this inherent limitation, the subsequent evolutionary step, ACURATE neo2, was introduced, focusing on reducing the PVL rate through a redesign of its structure.
The primary aim of this investigation was to assess the efficacy and safety profile of the ACURATE neo2 within a real-world patient cohort, both in the short and long term. Additionally, a comparative analysis of short-term postprocedural outcomes was conducted with its precursor, the ACURATE neo valve. To achieve this goal, patients scheduled for device implantation were prospectively recruited from five distinct medical centers located in Spain.
2 METHODS
2.1 Patient population and study design
The PRospective Evaluation Complementing Investigation with ACURATE neo Device (PRECISA) is an observational, retrospective, multicenter registry that enrolled a total of 296 patients (178 ACURATE neo, 118 ACURATE neo2) diagnosed with severe AS and requiring aortic valve prosthesis implantation, wherein either an ACURATE neo or ACURATE neo2 device was employed in accordance with the indications for use.
Initially, the study focused on the observational description of outcomes achieved with the ACURATE neo valve in a real-world population. However, in light of the unfavorable results observed with the ACURATE neo valve following the SCOPE I and SCOPE II studies, a new generation known as ACURATE neo2 was introduced to the European market. From October 2020 onwards, patients enrolled in the study received the new ACURATE neo2 valve. It was at this juncture that a descriptive study comparing the outcomes of the ACURATE neo valve against those of the new ACURATE neo2 prosthesis was undertaken.
Between January 2019 to September 2021 a consecutive cohort of patients was recruited from seven high-volume Spanish medical centers. No predefined exclusion criteria were established. Inclusion criteria encompassed adult individuals (aged 18 years or older) presenting with severe AS and requiring aortic valve prosthesis implantation. Both tricuspid and nontricuspid anatomies, as well as various access routes (e.g., trans-axillary, trans-apical, and trans-aortic), were considered for inclusion.
The Educación en Procedimientos de Intervencionismo en Cardiología (EPIC) Foundation served as the study's sponsor. Ethical approval for the study was obtained from the pertinent institutional review boards of the participating centers, and written informed consent was obtained from all enrolled patients.
2.2 Device
The ACURATE neo valve consists of a supra-annular porcine pericardial tissue valve with three leaflets, securely affixed within a self-expanding nitinol stent frame, designed for a two-step top-down implantation approach. Both the inner and outer surfaces of the stent body are enveloped by a pericardial sealing skirt. Notably, the ACURATE neo2 valve iteration has been enhanced with a 60% larger sealing skirt to reduce PVL.
The valves are delivered using the ACURATE neo delivery system, which can be introduced using the iSleeve 14 French expandable sheath. Three distinct valve sizes are available (S, M, and L), accommodating aortic annuli ranging from 21 to 27 mm.
2.3 End-points
The study endpoints were determined based on the criteria established by the Valve Academic Research Consortium-2 (VARC-2), which were the applicable standards during the study's inception.
The primary endpoint was defined as device success of implantation, characterized by the achievement of the following criteria: absence of procedural mortality, acurate positioning of a single prosthetic valve within the appropriate anatomical site, absence of prosthesis–patient mismatch, a mean aortic valve gradient < 20 mmHg, and the absence of moderate or severe prosthetic valve regurgitation.
Secondary endpoints included cardiovascular death, overall mortality, early safety outcomes, and valve safety outcomes at 30 days and 1 year. Early safety was ascertained using the VARC-2 definition, encompassing parameters such as all-cause mortality, all types of stroke (disabling and non-disabling), life-threatening bleeding, acute kidney injury of Stage 2 or 3, or necessitating renal replacement therapy, coronary artery obstruction necessitating intervention, major vascular complications, and valve-related dysfunction necessitating a repeat procedure. Valve safety was assessed as the proportion of patients experiencing structural valve deterioration requiring a repeat procedure (transcatheter or surgical heart valve replacement), valve-related dysfunction (defined by a mean aortic valve gradient ≥ 20 mmHg and/or moderate to severe prosthetic valve regurgitation), prosthetic valve endocarditis, prosthetic valve thrombosis, thromboembolic events (e.g., stroke), and VARC-defined bleeding events, unless distinctly unrelated to valve therapy.
2.4 Statistical analysis
Descriptive statistics were conducted for all variables within the total sample and stratified by valve type, detailing measures of central tendency and dispersion for quantitative variables, and absolute and relative frequencies for qualitative variables.
The statistical significance (p-value) of bivariate comparison between valve types for quantitative variables was calculated using the independent samples t-test when the distribution met normality assumptions, or the Mann–Whitney U test when normality assumptions were not met. For non-ordinal qualitative variables, the Fisher's exact test was consistently applied. The Spearman correlation test was used for assessing linear trends in ordinal variables.
In all cases, the normality of variable residuals was assessed using the Shapiro–Wilk test. If the obtained p-value (statistical significance) was less than 0.05, the null hypothesis of normality was rejected.
The effect of valve type on the three study objectives was assessed through multivariate analysis of variance (ANOVA), adjusting for the propensity score (PS) value of the ACURATE neo2 valve. Initially, the PS value for each patient was calculated using multivariate regression for valve type, adjusting for predictor variables (with p-value < 0.1). Subsequently, ANOVA was applied while controlling for PS and the other statistically significant variables related to the evaluated objective.
No adjustments were made to control for Type I error despite the presence of multiple comparisons.
Statistical analysis was performed using R statistical software version 4.3.0 (2023-04-21 ucrt).
3 RESULTS
The PRECISA registry successfully recruited a total of 296 patients across seven medical centers in Spain during the period spanning from January 2019 to September 2021. From January 2019 to September 2020, 178 patients were treated with the ACURATE neo valve. In September 2020, the ACURATE neo valve was replaced by the ACURATE neo2 device, which was subsequently used in this study. A total of 118 patients were treated with the ACURATE neo2 device until September 2021. Because of this change, although maintaining the study's primary and secondary endpoints, results are presented separately for both groups of patients.
3.1 Baseline characteristics and procedural data
The baseline clinical and echocardiographic characteristics of the study patients are presented in Tables 1 and 2. The mean age of the patients was 82 ± 6 years, with the majority being females (66.9%). Overall, there were no significant differences in the baseline clinical or echocardiographic characteristics among the patients.
| ACURATE neo (178) | ACURATE neo2 (118) | p | |
|---|---|---|---|
| Age, years | 82.0 (6.0) | 81.0 (5.7) | 0.06 |
| Sex | 0.38 | ||
| Female | 123 (69.1%) | 75 (63.6%) | |
| Male | 55 (30.9%) | 43 (36.4%) | |
| Symptoms | |||
| NYHA III or IV | 123 (81.1%) | 92 (92.9%) | 0.05 |
| Syncope | 7 (3.9%) | 8 (6.8%) | 0.29 |
| CCS grade III or IV | 6 (22.2%) | 2 (13.3%) | 0.54 |
| Body-mass index, kg/m2 | 27.7 | 28.5 | 0.053 |
| Predicted risk of mortality | |||
| STS | 4.9 (4.3) | 4.0 (4.2) | 0.17 |
| Euroscore II | 4.6 (4.8) | 3.9 (4.5) | 0.96 |
| Medical conditions and medical history | |||
| Diabetes | 81 (45.5%) | 45 (38.1%) | 0.23 |
| Hypertension | 149 (83.7%) | 94 (79.7%) | 0.43 |
| Hypercholesterolemia | 128 (71.9%) | 74 (62.7%) | 0.1 |
| Current smoker | 4 (2.2%) | 5 (4.2%) | 0.43 |
| Previous myocardial infarction | 15 (8.4%) | 10 (8.5%) | 1.00 |
| History of heart failure | 61 (34.3%) | 29 (24.6%) | 0.1 |
| Previous stroke or TIA | 11 (6.2%) | 15 (12.7%) | 0.06 |
| Extracardiac arteriopathy | 29 (16.3%) | 10 (8.5%) | 0.055 |
| Chronic kidney disease | 45 (25.3%) | 25 (21.2%) | 0.48 |
| Previous percutaneous coronary intervention | 31 (17.4%) | 26 (22.0%) | 0.37 |
| Previous coronary artery bypass grafting | 8 (4.5%) | 3 (2.5%) | 0.53 |
| Permanent pacemaker | 15 (8.4%) | 7 (5.9%) | 0.50 |
| History of AF or flutter | 44 (26.4%) | 34 (29.9%) | 0.88 |
| Bundle branch block | 36 (20.3%) | 15 (12.7%) | 0.29 |
| ACURATE neo (178) | ACURATE neo2 (118) | p | |
|---|---|---|---|
| Baseline echocardiographic characteristics | |||
| Aortic valve area | 0.8 (0.5) | 0.7 (0.5) | 0.41 |
| Maximum gradient | 74.5 (23.1) | 77.7 (20.3) | 0.28 |
| Mean gradient | 46.3 (15.1) | 47.4 (13.1) | 0.91 |
| Maximum velocity | 4.2 (0.8) | 4.2 (0.8) | 0.23 |
| Aortic insufficiency | 128 (71.9%) | 74 (62.7%) | 0.1 |
| None | 48 (27.0%) | 49 (41.5%) | |
| Mild | 89 (50.0%) | 48 (40.7%) | |
| Moderate | 28 (15.7%) | 20 (16.9%) | |
| Severe | 13 (7.3%) | 1 (0.8%) | |
| Aortic valve perimeter | 71.0 (11.6) | 70.9 (11.0) | 0.69 |
| Derived perimeter diameter | 20.2 (7.8) | 21.5 (5.7) | 0.68 |
| Bicuspid valve | 8 (5.1%) | 7 (6.8%) | 0.61 |
Some variations in procedural support were observed, which can be attributed to the evolution of the TAVI implantation procedure during the recruitment period. It is worth noting that at the time of valve implantation, the requirement for post-dilation was statistically higher in the ACURATE neo group (47.2%) compared to the ACURATE neo2 group (12.7%), with a p-value of <0.0001, without differences in the predilation rate, predilation balloon size, or valve size (Table 3).
| ACURATE neo (178) | ACURATE neo2 (118) | p | |
|---|---|---|---|
| Valve preparation | |||
| Previous valvuloplasty | 177 (99.4%) | 114 (96.6%) | 0.08 |
| Pre-dilatation balloon diameter | 21.4 (2.1) | 20.8 (2.8) | 0.09 |
| Valve size | 0.53 | ||
| S | 59 (33.1%) | 43 (36.4%) | |
| M | 74 (41.6%) | 48 (40.7%) | |
| L | 45 (25.3%) | 27 (22.9%) | |
| Need for post-dilatation | 84 (47.2%) | 15 (12.7%) | <0.0001 |
| Post-dilatation balloon diameter | 22.9 (2.0) | 24.0 (0.9) | 0.02 |
| Valve implantation results | |||
| Correct valve placement | 175 (98.3%) | 116 (98.3%) | 1.00 |
| Paravalvular aortic regurgitation | 0.0001 | ||
| None | 78 (43.8%) | 77 (65.3%) | |
| Grade I | 84 (47.2%) | 38 (32.2%) | |
| Grade II | 14 (7.9%) | 3 (2.5%) | |
| Grade III | 2 (1.1%) | 0 (0.0%) | |
| Mean transprosthetic gradient | 6.0 (3.9) | 4.9 (4.4) | 0.001 |
| Need for a second valve | 1 (0.6%) | 0 (0.0%) | 1.00 |
| Device malposition | 1 (0.6%) | 0 (0.0%) | 1.00 |
| Device embolization | 0 (0%) | 0 (0%) | 1.00 |
| Aortic ring rupture | 0 (0%) | 0 (0%) | 1.00 |
| Cardiac tamponade | 0 (0.0%) | 4 (3.4%) | 0.02 |
| Coronary occlusion | 0 (0%) | 0 (0%) | 1.00 |
| Intraprocedural death | 0 (0%) | 0 (0%) | 1.00 |
| Need for cardiac surgery | 0 (0%) | 0 (0%) | 1.00 |
3.2 Primary and secondary end-points
The primary endpoint occurred in 43 out of the 296 patients included in the study (14.5%). The ACURATE neo group showed a significantly higher risk of reaching the primary endpoint compared to the ACURATE neo2 group (18% vs. 9.3%), as evidenced by both traditional regression analysis (p = 0.04) and PS analysis (p = 0.01). (Table 4). This was primarily attributed to the rate of significant aortic insufficiency at discharge (9.8% vs. 5%, p = 0.03) (Figure 1), a difference that persisted at both the 30-day (p = 0.04) and 12-month follow-up (p = 0.05).
| ACURATE neo (178) | ACURATE neo2 (118) | p | |
|---|---|---|---|
| Primary objective | 32 (18.0%) | 11 (9.3%) | 0.04 |
| Intraprocedural death | 0 (0%) | 0 (0%) | 1.00 |
| In-hospital death | 5 (2.8%) | 0 (0.0%) | 0.16 |
| 30-day mortality | 3 (1.8%) | 1 (0.9%) | 0.65 |
| Device malposition | 1 (0.6%) | 0 (0.0%) | 1.00 |
| Mean transvalvular gradient > 20 mmHg | 2 (1.2%) | 2 (1.7%) | 1.00 |
| Paravalvular aortic regurgitation ≥ II | 17 (9.8%) | 6 (5%) | 0.03 |
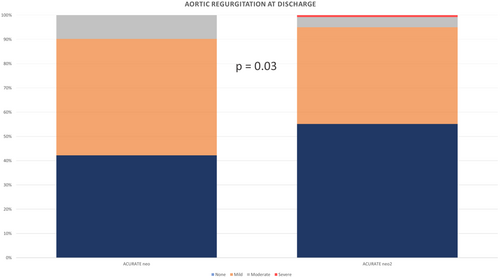
Additionally, there was an increased risk of in-hospital mortality in the ACURATE neo group compared to its successor, although this difference was not statistically significant (2.8% vs. 0%, p = 0.16).
There were no instances of device embolization, ring rupture, coronary occlusion, the necessity for emergency cardiac surgery, or intraprocedural mortality. Only one occurrence of valve malposition was documented, and it occurred during a transaxilar TAVI procedure using the ACURATE neo device. This event required the successful implantation of a second valve (valve-in-valve) to correct the malposition.
Regarding the secondary objectives at discharge, it is worth noting a better functional class in the ACURATE neo2 group compared to its predecessor (p = 0.001) and a slightly higher mean transvalvular gradient measured by echocardiography (8.8 vs. 7.9 mmHg, p = 0.03).
In reference to the events that occurred during the follow-up, although they consistently happened more frequently in the ACURATE neo group compared to ACURATE neo2, statistically significant differences were not observed at 30 days (Table 5) nor at 1 year (Table 6).
| ACURATE neo (178) | ACURATE neo2 (118) | p | |
|---|---|---|---|
| Secondary objectives (30-day follow-up) | 41 (23.0%) | 22 (18.6%) | 0.39 |
| Intraprocedural death | 0 (0%) | 0 (0%) | 1.00 |
| In-hospital death | 5 (2.8%) | 0 (0.0%) | 0.16 |
| 30-day mortality | 3 (1.8%) | 1 (0.9%) | 0.65 |
| Stroke at discharge | 5 (1.7%) | 1 (0.8%) | 0.31 |
| Transient ischemic attack at discharge | 0 (0%) | 1 (0.8%) | 0.39 |
| 30-day stroke | 0 (0%) | 1 (0.9%) | 0.47 |
| 30-day transient ischemic attack | 0 (0%) | 0 (0%) | 1.00 |
| Coronary occlusion at discharge | 1 (0.6%) | 0 (0%) | 1.00 |
| 30-day coronary occlusion | 0 (0%) | 0 (0%) | 1.00 |
| Major bleeding at discharge | 6 (3.4%) | 5 (4.2%) | 0.76 |
| 30-day major bleeding | 0 (0%) | 0 (0%) | 1.00 |
| Kidney failure at discharge | 6 (3.4%) | 5 (4.2%) | 0.76 |
| Mean Ao gradient ≥20 mmHg at discharge | 2 (1.2%) | 2 (1.7%) | 1.00 |
| 30-day mean Ao gradient ≥20 mmHg | 0 (0%) | 1 (4.2%) | 1.00 |
| Aortic valve regurgitation ≥ moderate at discharge | 17 (9.8%) | 6 (5%) | 0.03 |
| 30-day aortic valve regurgitation ≥ moderate | 7 (4%) | 3 (2.5%) | 0.04 |
| ACURATE neo (178) | ACURATE neo2 (118) | p | |
|---|---|---|---|
| Secondary objectives (1-year follow-up) | 67 (37.6%) | 38 (32.2%) | 0.39 |
| 1-year mortality | 17 (9.6%) | 11 (9.3%) | 1.00 |
| 1-year stroke | 9 (5%) | 1 (0.8%) | 0.2 |
| 1-year coronary occlusion | 1 (0.6%) | 0 (0%) | 1.00 |
| 1-year major bleeding | 11 (6.5%) | 6 (5.1%) | 0.75 |
| 1-year mean Ao gradient ≥20 mmHg | 4 (2.3%) | 4 (3.3%) | 1.00 |
| 1-year aortic valve regurgitation ≥ moderate | 39 (21.9%) | 16 (13.6%) | 0.047 |
3.3 Comparison between trans-femoral and non-transfemoral procedures
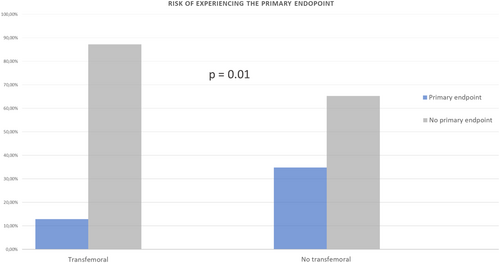
When comparing the group of patients with primary transfemoral access (92.3%) to the group with non-transfemoral primary access (7.7%, including trans-axillary [7.1%] and trans-apical [0.6%] accesses), a statistically significant increase in the risk of reaching the primary endpoint was observed in the non-transfemoral group (34.8% vs. 12.8%, p = 0.01) (Figure 2). Likewise, the risk of experiencing at least moderate aortic insufficiency at discharge was higher in the non-transfemoral access group (26.1% vs. 6.2%, p = 0.01). The non-transfemoral access group also had the sole case of valve malposition and accounted for 60% of all major vascular complications, although these differences were not statistically significant.
4 DISCUSSION
In broad terms, the efficacy and safety outcomes of both ACURATE valve prosthesis were satisfactory, with a high rate of device implantation success and a low risk of complications, results that were maintained at discharge and during the 1-year follow-up period. As the ACURATE neo2 replaced the ACURATE neo device during the study period, this provided an opportunity to compare the results between the first and second generations of this transcatheter aortic valve.
Two important conclusions can be drawn from this study. The first, consistent with other previously published findings, is that the ACURATE neo2 valve retains the same advantages as the first generation, including its high success rate and low complication rate, but has successfully addressed its main drawback: PVL. The second conclusion, not previously published, is that the long-term clinical and hemodynamic outcomes of the ACURATE neo2 valve are excellent, with a low rate of all-cause mortality and valve-associated complications.
The main change in the design of the ACURATE neo2 valve compared to its predecessor is the increased size of the skirt, with minor changes in the frame design or in enhancing its radial strength. This explains why events not directly dependent on the skirt do not show significant changes and, in the case of ACURATE valves, demonstrate excellent outcomes. No patient in our study experienced valve embolization, aortic ring rupture, or coronary occlusion. Similarly, the rate of need for a pacemaker was low, comparable between both groups, and consistent with previously published literature.6, 7
In terms of transvalvular gradients, ACURATE neo exhibits lower values due to their supra-annular design compared to intra-annular valves. Additionally, they demonstrate reduced rates of prosthesis–patient mismatch, larger effective orifice areas, and a lower risk of valve thrombosis compared to the SAPIEN 3 device.8, 9
In this study, a subtle increase was observed in the ACURATE neo2 compared to the neo (7.9 mmHg vs. 8.8 mmHg, p = 0.03), as has been noted in other studies conducted with this valve.10-12 Despite the discrepancy between both models observed in this study, the mean gradients of the ACURATE neo2 valve remain very low, akin to those observed in other studies of the ACURATE neo, and notably lower than the gradients recorded in balloon-expandable valves, with no instances of prosthesis–patient mismatch and a low rate of a mean aortic valve gradient ≥ 20 mmHg at discharge in both groups.
On the other hand, the main limitation of the ACURATE neo pertains to the risk of paravalvular leak, which is comparatively higher when compared to the SAPIEN 3 and Evolut R.7 4, 5 This consideration holds paramount importance as post-procedural aortic regurgitation has been significantly associated with long-term survival following TAVI.13 To address this concern, the new ACURATE neo2 valve features a taller outer pericardial skirt.
In our study, patients who underwent TAVR using the ACURATE neo2 device experienced significantly lower rates of intraprocedural and long-term postprocedural aortic regurgitation compared to those who underwent TAVR using the ACURATE neo device, a finding supported by other studies,10, 11 although some have not demonstrated statistically significant differences.12
However, while the PVL rate of the ACURATE neo2 valve in this study is lower than that of the ACURATE neo, it is slightly higher than reported in other similar studies.10, 11, 14, 15 Several reasons may explain these findings. First, this study reflects the initial experience of multiple centers with the ACURATE neo2 valve. Second, a low rate of post-dilation (12.7%) was observed in our study compared to the previously cited studies (30%–40% post-dilation). While this may explain a higher PVL rate, it could also be attributed to the exceptionally low rate of periprocedural complications (such as ring rupture, the need for pacemaker implantation, and embolization) typically associated with post-dilation and valve oversizing. Third, there was a tendency to utilize smaller prostheses in this study compared to others (36.44% S, 40.68% M, and 22.8% L), although the cover index (6.91%) was similar to these studies. This could be explained by a tendency to implant the ACURATE neo2 valve in patients who potentially stand to benefit more from this valve compared to others, predominantly women with a small aortic annulus.
Fourth, this study included patients with axillary and transapical access, which, despite being a minority, are associated with excess complications and PVL compared to transfemoral access. This may have adversely affected the overall outcome when compared to other studies where the rate of non-transfemoral access is very low or excluded.
Lastly, it is worth noting that the PVL rate at discharge decreased at 30 days (from 5.1% to 2.5%), and none of the patients with significant PVL at discharge died during the 12-month follow-up period.
In terms of long-term follow-up, upon assessing the clinical and hemodynamic data of the ACURATE neo2 valve at 1 year, we observe excellent results. The all-cause mortality rate at this juncture is 9.3%, slightly surpassing other studies with similar populations, despite a higher rate of PVL. Moreover, valve-associated events such as coronary occlusion, valve degeneration, and prosthetic thrombosis are exceedingly rare. The rate of PVL remains low at 5.1% and shows no increase compared to the discharge rate.
These results confer to the ACURATE neo2 valve a profile of high success rate with excellent safety, both in the short and long term, and a contained rate of PVL. Therefore, the ACURATE neo2 valve appears to be an excellent upgrade from the first version, retaining the advantages and correcting the shortcomings of the old one. It positions itself as an excellent candidate that should be compared in future clinical trials with the leading valves in the field.
5 CONCLUSIONS
In our study, the ACURATE transcatheter aortic valve was associated with excellent clinical results and an extremely low rate of complications in both the short and long term. PVL, the main limitation of the ACURATE neo, has been clearly improved with the novel design of the ACURATE neo2.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.