Solid state, pulsed-wave 355 nm UV laser atherectomy debulking in the treatment of infrainguinal peripheral arterial disease: The Pathfinder Registry
Abstract
Background
Atherectomy is an important option for debulking atherosclerotic plaque from diseased arteries in patients with infrainguinal arterial disease. Laser atherectomy uses a high-powered laser to remove the plaque from the arteries to restore blood flow.
Aims
The Pathfinder multicenter registry was initiated to evaluate the safety and efficacy of the 355 nm laser atherectomy system in a real-world setting for the treatment of de novo, re-stenotic and in-stent restenosis (ISR) lesions in infrainguinal arteries of patients with peripheral artery disease (PAD).
Methods
The study was a prospective, single-arm, multicenter, open-label registry study for patients treated with the 355 nm laser system. Clinical and lesion characteristics, procedural safety and efficacy data, and baseline, 6-, and 12-month outcomes data, including Ankle Brachial Index (ABI), Rutherford class, and Walking Impairment Questionnaires (WIQ), were collected. The primary efficacy endpoint was the achievement of ≤30% final residual stenosis at the index lesion postatherectomy and adjunctive therapy evaluated by an angiographic Core Lab. The primary safety endpoint was the percentage of subjects who did not experience periprocedural major adverse events (PPMAEs) before discharge.
Results
One hundred and two subjects with 121 lesions treated with the 355 nm laser device at 10 centers were included in the analysis. Mean age was 68.4 ± 10.21 years, 61.8% of subjects were male, 44.6% had critical limb ischemia (CLI), and 47.3% had tibial lesions. The mean residual stenosis at the end of the procedure was 24.4 ± 15.5 with 69 lesions (69.0%) achieving technical procedural success (<30% stenosis); similar rates were observed for subjects with ISR (25.5 ± 14.9), chronic total occlusion (CTO) (28.1 ± 17.0), and severe calcification (36.5 ± 21.6) lesions. Mean ABI, Rutherford, and WIQ scores were improved at both 6 and 12 months. Ninety-seven of 102 subjects (95.1%) met the primary safety endpoint of not experiencing a PPMAE before discharge.
Conclusions
The initial data from the Pathfinder Registry demonstrates the 355 nm laser system is safe and effective in a real-world setting for performing atherectomy in patients with infrainguinal PAD.
Abbreviations
-
- ABI
-
- Ankle Brachial Index
-
- CD-TLR
-
- clinically driven target lesion revascularization
-
- CLI
-
- critical limb ischemia
-
- CTO
-
- chronic total occlusion
-
- ISR
-
- in-stent restenosis
-
- MAE
-
- major adverse event
-
- NHLBI
-
- National Heart, Lung and Blood Institute
-
- PAD
-
- peripheral artery disease
-
- PARC
-
- Peripheral Academic Research Consortium
-
- PPMAE
-
- periprocedural major adverse event
-
- TASC
-
- Trans-Atlantic Inter-Society Consensus
-
- TBI
-
- Tibial Brachial Index
-
- WIQ
-
- Walking Impairment Questionnaire
1 INTRODUCTION
Peripheral artery disease (PAD) is a common atherosclerotic vascular pathology that affects both quality of life and life expectancy with an increased risk of cardiovascular events.1 Although the majority of patients with PAD are asymptomatic, patients may present with intermittent claudication acute or chronic limb-threatening ischemia (CLTI) and an increased risk of amputation.2 Revascularization, percutaneous or surgical, is performed for patients with ALI, CLTI, and those with limiting claudication that failed medical therapy and exercise.3-5
Atherectomy6-8 has emerged as an important plaque modification strategy for flow restoration and facilitation of angioplasty/stenting in patients with complex infrainguinal arterial disease, and has been shown to reduce flow-limiting dissections and bailout stenting.9 Recently, several studies have shown a benefit of atherectomy before use of standard or drug-coated balloon angioplasty.9-11
The Auryon System (AngioDynamics, Inc.) is an atherectomy device indicated for the treatment of infrainguinal arterial stenoses and occlusions, including in-stent restenosis (ISR).12 The Auryon System combines a catheter using 355 nm Nd:YAG laser technology with a short pulse between 10 and 25 ns, and a blunt blade enabling safe and efficient atherectomy without thermal injury to the treated artery. The 2.0 and 2.35 mm catheters can also be used to aspirate thrombus within occlusive, native, and stented femoropopliteal arteries. Prior data have demonstrated effectiveness of the 355 nm laser for debulking of several plaque morphologies including fibrotic, fibrocalcific, and moderate-to-severe calcified lesions.13, 14
We present here an initial report of the 12 months findings from the Pathfinder Registry, a real-world study evaluating the safety and performance of the 355 nm laser system to treat de novo, re-stenotic, and ISR lesions in infrainguinal arteries of subjects with PAD (Clinicaltrials.gov NCT04229563).
2 PATIENTS AND METHODS
2.1 Study design
The Pathfinder Registry was a prospective, single-arm, multicenter, open-label, observational clinical study assessing the safety and efficacy of the 355 nm laser system in subjects with symptomatic infrainguinal PAD at 10 sites in the United States between August 2020 and March 2021. Institution Review Board (IRB) approval was obtained from a central IRB and all subjects included in the registry signed informed consents before enrollment.
Inclusion criteria were subjects 18 years of age or older with symptomatic infrainguinal PAD. All subjects had documented symptomatic atherosclerotic PAD with claudication or CLTI (Rutherford Classification 2–5). Subjects were excluded if a target lesion was in an bypass graft; there was planned use of another atherectomy device during the same procedure; if the subject was participating in another study using an interventional device that did not have regulatory clearance; if they had comorbid condition(s), which could limit the subject's ability to participate in the trial or to comply with follow-up requirements; or if the subject had anticipated survival of <1 year.
2.2 Endpoints
The primary efficacy endpoint for the study was technical procedural success measured as the percentage of subjects with successful revascularization of target vessel following the use of the 355 nm laser System and any adjunctive treatment if required, including angioplasty and stent placement at the operator's discretion. Successful revascularization was defined as achieving ≤30% residual stenosis at the index lesion postatherectomy and adjunctive therapy as evaluated by an angiographic Core Lab. The primary safety endpoint was the percentage of subjects who did not experience periprocedural major adverse events (PPMAEs) before discharge. PPMAEs included flow-limiting dissection (National Heart, Lung and Blood Institute [NHLBI] grade > C), clinically significant distal embolization which required more than simple aspiration or a vasodilator, the need for bailout stenting defined as being due to either a flow-limiting dissection per NHLBI grade > C or residual narrowing of >30%, a major or unplanned amputation, target vessel perforation requiring endovascular or surgical repair, or death. Supporting Information S1: Table 1 lists the secondary endpoints for the study.
2.3 Device
The 355 nm laser consists of a single-use catheter containing an array of optical fibers with a blunt rounded blade at its tip to both penetrate and transmit 355 nm coherent laser energy to modify and ablate atherosclerotic plaque. The laser and the blade work in synergy and the unique laser energy delivery profile enables a higher affinity to lesional material than the endothelium of blood vessels, inherently reducing the risk for vessel injury.15, 16 The 355 nm laser catheter is connected to the laser generator and transmits 40 short pulses per second to the occluded or narrowed artery at preset fluency levels of 50 or 60 mJ/mm2. The system has four different single-use catheters with diameters ranging from 0.9 to 2.35 mm. The 2 and 2.35 mm catheters have an aspiration feature intended for debris and thrombus collection and removal from the vessel during the atherectomy procedure. The 2.35 mm catheter also has an “off-center” feature which can facilitate debulking of eccentric lesions. All catheters are inserted over a 300 cm 0.014” guide wire that has been used to cross the lesion intraluminally.
2.4 Study methods
Baseline data were collected to characterize subjects in terms of comorbidities, disease stage, prior treatments, lesion/occlusion baseline characteristics, and details of the atherectomy procedure and the adjunctive therapy. Ankle Brachial Index (ABI) and Rutherford classification were determined, and a Walking Impairment Questionnaire (WIQ)8 was administered pre-procedurally and at 6, 12, and 24 (±1) months following the procedure.
2.5 Study procedure
All subjects underwent atherectomy with the 355 nm laser system. Adjunctive therapy with either standard balloon angioplasty or drug-coated balloon angioplasty and stenting were used per operator discretion.10, 17, 18 Stent placement was limited and at the discretion of the operator. Prospective data, including MAEs, lesion patency rate, and clinical outcomes were collected to evaluate the clinical course of the subjects during the study follow-up period. Procedural angiograms were evaluated by an independent angiographic Core Laboratory (Prairie Education and Research Cooperative d/b/a Synvacor), to determine target vessel(s), target lesion length, diameter stenosis percentage, minimal lumen diameter, and tibial run-off(s). Angiographic films that were captured and submitted in accordance with the predefined angiographic acquisition protocol were qualitatively and quantitatively analyzed using a validated QVA software program (Cardiovascular Angiographic Analysis System). The analysis was done by two different readers, except in cases when values were found inconsistent or problems were identified, then an overread by a third reader or the medical director review was required. All data were collected using an electronic data collection platform. Subjects were managed per each site's standard clinical practices and the 355 nm laser system was utilized per the manufacturer's instructions.
2.6 Statistical analysis
Statistical analysis was performed by the study sponsor. The statistical analyses conducted for quantitative variables included frequency counts and percentages, means, SD, minimums, and maximums of each parameter. Categorical variables were summarized by frequencies and percentages. Unless explicitly stated, percentages utilized a denominator corresponding to the number of unique subjects or lesions that contributed to the endpoint. Statistical analyses were run using SAS Software version 9.4 (SAS Institute Inc.).
An α of 0.05 was used to determine statistical significance.
3 RESULTS
3.1 Patient population
One hundred and forty-two subjects were consented for enrollment in the study. Forty subjects were excluded from the study, 35 due to the absence of angiographic disease, three did not meet the inclusion/exclusion criteria, and two did not have a proper signed informed consent form. One subject inadvertently enrolled in the study was classified as Rutherford Class 6 and this data was included in the analysis since proper consent had been received and the patient underwent treatment with the 355 nm laser device and had data collected during follow-up visits.
3.2 Baseline demographics and clinical characteristics
Baseline demographics and clinical characteristics of the 102 subjects with one hundred and twenty-one 355 nm laser-treated target lesions in the intent-to-treat study population are listed in Table 1. Mean age was 68.4 ± 10.21 years, ranging from 28 to 92 years. There were more males than females (61.8% vs. 38.2%), with most subjects reported as being Caucasian (71.6%). Current and former smokers represented 28.4% and 38.2% of the study population, respectively. There was a high prevalence of one or more comorbidities, including hypertension (87.3%), hyperlipidemia (75.5%), diabetes mellitus (52.9%), and coronary artery disease (44.1%). Sixty of the 102 subjects (58.8%) had undergone a previous surgical or percutaneous intervention to treat PAD (46 [45.1%] were in the index limb). The average time since the last PAD intervention was 2.3 ± 3.28 years ago, with 39 of these 60 subjects (38.2%) having had an atherectomy; 46 (76%) of prior interventions were in the index limb. The mean ABI (or Tibial Brachial Index [TBI] if ABI was not available) was 0.73 ± 0.28 (range 0.28–1.41, n = 68). The mean Rutherford was 3.6 ± 0.92. The mean overall WIQ score at baseline for the 95 subjects with available data was 22.8 ± 22.5 (range 0.1–96.4) with 82.1% of subjects deemed to be low performers due a to overall WIQ score of 39 or less.19
| Age, years (mean ± SD) | 68.4 ± 10.2 |
| Sex, n (%) | |
| Male | 63 (61.8%) |
| Female | 39 (38.2%) |
| Race, n (%) | |
| American Indian or Alaska Native | 2 (2.0%) |
| Black or African American | 14 (13.7%) |
| Caucasian | 73 (71.6%) |
| Other | 13 (12.7%) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 21 (20.6%) |
| Not Hispanic or Latino | 65 (63.7%) |
| Not Reported | 10 (9.8%) |
| Unknown | 6 (5.9%) |
| BMI, kg/m2 (mean ± SD) | 28.8 ± 5.4 |
| Smoking history, n (%) | |
| Current | 29 (28.4%) |
| Former | 39 (38.2%) |
| Never | 34 (33.3%) |
| Comorbiditiesa | |
| CAD | 45 (44.1%) |
| MI | 13 (12.7%) |
| CRF | 2 (2.0%) |
| Hyperlipidemia | 77 (75.5%) |
| Hypertension | 89 (87.3%) |
| Past stroke or TIA | 14 (13.7%) |
| Angina | 19 (18.6%) |
| CHF | 11 (10.8%) |
| CKD | 9 (8.8%) |
| Diabetes mellitus | 54 (52.9%) |
| Other | 20 (19.6%) |
| Past interventions associated (PAD), n (%) | 60 (58.8%) |
| POBA | 37 (36.3%) |
| DCB | 14 (13.7%) |
| BMS | 31 (30.4%) |
| DES | 9 (8.8%) |
| Bypass | 2 (2.0%) |
| Graft | 0 (0.0%) |
| Atherectomy | 39 (38.2%) |
| Other | 14 (13.7%) |
| Time from the last PAD intervention to screening visit, years (mean ± SD) | 2.3 ± 3.3 |
| Target limb ABI/TBIb at baseline, n = 68 (mean ± SD) | 0.73 ± 0.28 |
| <0.50 | 12 (17.6%) |
| 0.50–0.79 | 31 (45.6%) |
| 0.80–0.89 | 6 (8.8%) |
| 0.90–1.30 | 15 (22.1%) |
| >1.30 | 4 (5.9%) |
| Rutherford classification at baseline, n = 101 (mean ± SD) | 3.64 ± 0.92 |
| 2 | 5 (5.0%) |
| 3 | 51 (50.5%) |
| 4 | 21 (20.8%) |
| 5 | 23 (22.8%) |
| 6 | 1 (1.0%)e |
| WIQ overall scorec at baseline, n = 95 (mean ± SD) | 22.77 ± 22.52 |
| WIQ overall scorec at baseline, by category,d n (%) | |
| ≤39% (low performer) | 78 (82.1%) |
| >39% | 17 (17.9%) |
- Abbreviations: ABI, Ankle Brachial Index; BMI, body mass index; BMS, bare metal stent(s); CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; CRF, chronic renal failure; DCB, drug-coated balloon(s); DES, drug-eluted stent(s); MI, myocardial infarction; PAD, peripheral artery disease; POBA, plain old balloon(s); TBI, Tibial Brachial Index; TIA, transient ischemic attack; WIQ, Walking Impairment Questionnaire.
- a Subjects can have more than one comorbidity.
- b When ABI was not obtained, TBI was considered instead.
- c The WIQ Overall Score was computed as mean of Walking Distance, Walking Speed, and Stair Climbing Scores; it was reported as missing if any of the three scores were missing.
- d Threshold for low performer based on Sagar et al.19
- e Even though an exclusion criteria, subject was included in the analysis, as patient had proper informed consent and was treated with the 355 nm laser device.
3.3 Baseline angiographic variables
Baseline Core Lab data were available for 107 of the 121 (88.4%) target lesions. Treated lesion locations are shown in Table 2. De novo stenosis was the most common stenosis type (67.8%), followed by ISR (22.3%) and non-ISR (17.4%). Lesions had a mean Core Lab-reported length of 13.6 ± 11.5 cm (range 0.5–52.0 cm) and mean percent diameter stenosis of 87.1 ± 16.5 (range 17–100). Over a third of the lesions (36.5%) were reported by the Core Lab to have moderate-to-severe calcification, per Peripheral Academic Research Consortium (PARC) grading system with circumferential calcium distribution of ≥180° (both sides of vessel) and >50% of the lesion length.20
| Core Lab-reported lesion locationab | |
| CFA | 14 (13.0%) |
| SFA | 55 (50.9%) |
| Popliteal artery | 37 (34.3%) |
| AT | 26 (24.1%) |
| PT | 13 (12.0%) |
| Peroneal | 3 (2.8%) |
| Dorsalis pedis | 6 (5.6%) |
| Tibioperoneal trunk | 3 (2.8%) |
| Site-reported stenosis typea (n = 121) | |
| De novo | 82 (67.8%) |
| Restenosis | 21 (17.4%) |
| ISR | 27 (22.3%) |
| Core Lab-reported pre-355 nm laser target lesion length, cm (mean ± SD) | 13.6 ± 11.5 |
| Core Lab-reported reference vessel diameter, mm (mean ± SD) | 3.83 ± 1.410 |
| Chronic total occlusion | 48 (44.4%) |
| Core Lab-reported calcification assessment (PARC) | |
| 0—None | 31 (29.0%) |
| 1—Focal | 4 (3.7%) |
| 2—Mild | 19 (17.8%) |
| 3—Moderate | 22 (20.6%) |
| 4—Severe | 17 (15.9%) |
| Unknown | 14 (13.1%) |
| Core Lab-reported TASC lesion type | |
| TASC A | 48 (44.9%) |
| TASC B | 24 (22.4%) |
| TASC C | 16 (15.0%) |
| TASC D | 19 (17.8%) |
| Unknown | 0 (0.0%) |
| Site-reported tortuosity (n = 121) | |
| None | 67 (55.4%) |
| Mild | 31 (25.6%) |
| Moderate | 16 (13.2%) |
| Severe | 7 (5.8%) |
- Note: N = 121 for site-reported data and n = 107 for Core Lab-reported data.
- Abbreviations: AT, anterior tibial; CFA, ommon femoral artery; ISR, in-stent restenosis; PARC, Peripheral Academic Research Consortium; PT, posterior tibial; SFA, superficial femoral artery; TASC, Trans-Atlantic Inter-Society Consensus.
- a More than one entry is possible (since some patients had longer lesions), N > 100%.
- b Lesions can involve several arteries.
3.4 Procedural data
One hundred and fifty-four lesions were treated with or without 355 nm laser system with a mean of 1.5 ± 0.77 (range 1–4) lesions treated per patient (Supporting Information S1: Table 2). This included 121 lesions treated with the 355 nm laser system and 33 lesions that were not treated with atherectomy before the use of angioplasty balloons or stents. One or more adjunctive therapies were used after atherectomy in 114 of the 120 (data were missing for one patient) lesions (95.0%), including balloon angioplasty in 104 (86.0%) and stents in (28) 23.1% of lesions. Other adjunctive therapies are available in Supporting Information S1: Table 2. Mean procedure time was 87.5 ± 39.5 min.
All four commercially available laser catheter sizes were used during procedures, with a mean laser duration of 123.3 ± 87.97 s.
3.5 Efficacy endpoints
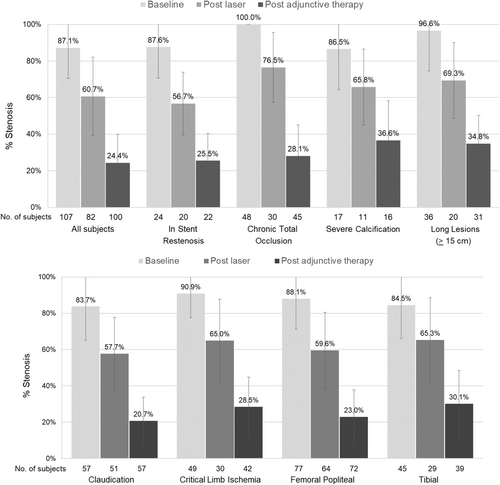
Table 3 displays the angiography Core Lab-reported findings. The final residual diameter stenosis, following the use of the 355 nm laser system and any final adjunctive treatment (if required), was 24.4 ± 15.5. Sixty-nine lesions (69.0%) achieved the primary effectiveness endpoint of technical procedural success defined as ≤30% residual stenosis at the index lesion postatherectomy and adjunctive therapy, as evaluated by an angiographic Core Lab. Figure 1 shows improvements in stenosis diameter from baseline following both 355 nm laser treatment and subsequent adjunctive therapy for all subjects and in subgroup analyses of subjects with ISR, chronic total occlusion (CTO), and with Core Lab-defined severe calcification at baseline.
| Number of lesions (mITT) | Baseline (pre-355 nm laser) N = 107 |
Post-355 nm laser treatment N = 84 |
Post final treatment (355 nm laser TL only) N = 103 |
Post final treatment (total treated) N = 103 |
|---|---|---|---|---|
| Target lesion length (cm) | ||||
| N | 107 | 82 | 100 | 97 |
| Mean ± SD | 13.59 ± 11.512 | 12.94 ± 9.855 | 13.79 ± 10.678 | 20.19 ± 13.039 |
| Median [Q1, Q3] | 10.75 [4.07, 20.00] | 10.67 [6.00, 17.40] | 11.09 [5.61, 19.16] | 16.23 [9.59, 29.03] |
| (Min, Max) | (0.51, 52.00) | (1.03, 45.53) | (1.07, 46.13) | (2.02, 51.42) |
| Reference vessel diameter (RVD, mm) | ||||
| N | 101 | 81 | 100 | 97 |
| Mean ± SD | 3.83 ± 1.410 | 4.01 ± 1.321 | 4.27 ± 1.490 | 4.21 ± 1.459 |
| Median [Q1, Q3] | 4.09 [2.77, 4.76] | 4.12 [2.97, 4.81] | 4.63 [2.94, 5.34] | 4.42 [2.97, 5.28] |
| (Min, Max) | (0.00, 7.34) | (1.40, 7.96) | (1.24, 7.45) | (1.24, 7.24) |
| MLD (mm) | ||||
| N | 107 | 82 | 100 | 97 |
| Mean ± SD | 0.54 ± 0.779 | 1.63 ± 1.041 | 3.28 ± 1.391 | 3.08 ± 1.304 |
| Median [Q1, Q3] | 0.14 [0.00, 0.81] | 1.65 [0.82, 2.13] | 3.38 [2.05, 4.28] | 3.29 [1.99, 3.98] |
| (Min, Max) | (0.00, 4.11) | (0.00, 4.18) | (0.31, 6.65) | (0.31, 5.98) |
| % diameter stenosis | ||||
| N | 107 | 82 | 100 | 97 |
| Mean ± SD | 87.1 ± 16.58 | 60.7 ± 21.37 | 24.4 ± 15.48 | 27.5 ± 15.68 |
| Median [Q1, Q3] | 96.0 [75.0, 100.0] | 57.5 [45.0, 75.0] | 23.5 [13.5, 32.0] | 27.0 [18.0, 33.0] |
| (Min, Max) | (17, 100) | (13, 100) | (0, 79) | (0, 79) |
| Run-off vesselsa | ||||
| AT | 50 (46.7%) | 26 (31.0%) | 58 (56.3%) | |
| PT | 60 (56.1%) | 33 (39.3%) | 66 (64.1%) | |
| Peroneal | 50 (46.7%) | 24 (28.6%) | 51 (49.5%) | |
| Not done | 16 (15.0%) | 35 (41.7%) | 20 (19.4%) | |
| Arch | ||||
| N | 107 | 84 | 103 | |
| Complete | 23 (21.5%) | 8 (9.5%) | 34 (33.0%) | |
| Incomplete | 56 (52.3%) | 29 (34.5%) | 38 (36.9%) | |
| Not done | 28 (26.2%) | 47 (56.0%) | 31 (30.1%) | |
| Flow assessment | ||||
| N | 107 | 84 | 103 | |
| Brisk | 51 (47.7%) | 64 (76.2%) | 87 (84.5%) | |
| Slow | 3 (2.8%) | 6 (7.1%) | 1 (1.0%) | |
| No flow | 42 (39.3%) | 7 (8.3%) | 0 (0.0%) | |
| Unknown | 11 (10.3%) | 7 (8.3%) | 15 (14.6%) | |
- Abbreviations: AT, anterior tibial; mITT, modified intent-to-treat; MLD, minimal lumen diameter; N, number of non-missing data; PT, posterior tibial.
- a More than one entry was possible.
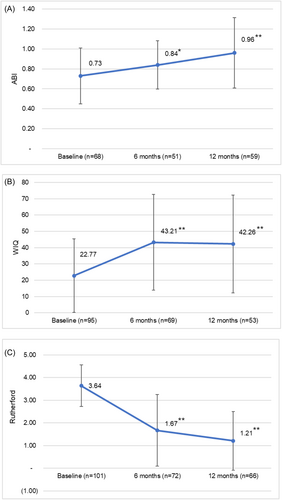
Although the study was not powered to assess differences in ABI, WIQ, or Rutherford classification, mean ABI (or TBI if ABI was not available) was found to be significantly increased versus baseline at both 6 months (p = 0.0180) and 12 months (p < 0.0001) following the procedure (Figure 2A). WIQ scores were also significantly increased at 6 and 12 months (p < 0.0001) (Figure 2B). Rutherford classification significantly decreased from baseline during the same time intervals (p < 0.0001) (Figure 2C) with 94% of subjects showing improvement at 12 months compared to baseline.
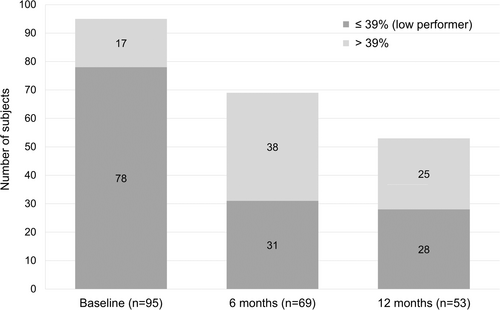
The percentage of subjects rated as low performers on the WIQ (overall score of ≤39) decreased from 82.1% at baseline to 37.2% at 6 months and 29.3% at 12 months (Figure 3). The percentage of subjects with duplex ultraound (DUS)assessed patency, defined as less than 50% stenosis, remained consistent from the 6-month through the 12-month follow-up periods ranging from 71.0% at 6 months to 71.6% at 12 months (Figure 4). At 6- and 12-month DUS follow-up, 17 (74.0%) and 15 (65.2%) of below the knee lesions (BTK)remained free from occlusions, whereas the rates of above the knee lesions were 43 (87.8%) and 38 (80.9%), respectively.
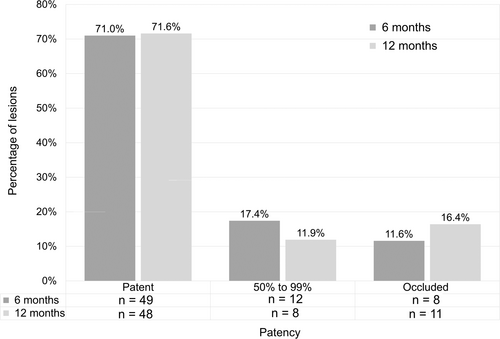
3.6 Safety endpoints
Ninety-seven subjects of the 102 subjects (95.1%) met the primary safety endpoint of not experiencing a PPMAE before discharge. PPMAEs occurred in five (4.9%) subjects. This included one subject who developed a clinically significant distal embolization following the use of the 355 nm laser device, which resolved intraprocedurally with thrombectomy and stenting, without further complications. Four (3.9%) subjects required bailout stenting following adjunctive therapy with balloon angioplasty. This included two (2.0%) subjects who experienced type C dissections revealed after balloon inflation and two (2.0%) who remained with residual narrowing. The need for bailout stenting occurred only after balloon angioplasty was performed with no patients requiring stenting after the initial treatment with the 355 nm laser device before angioplasty.
There were no periprocedural deaths, major or unplanned amputations, or target vessel perforations requiring endovascular or surgical repair before discharge. Only one PPMAE (drug-eluted stent[s] bailout stenting due to >30% stenosis postballoon angioplasty in the target lesion) was deemed by the physician to be related to the 355 nm laser system, resulting in 99.0% of subjects meeting the secondary safety endpoint. Among all 102 subjects, only one patient received a filter as an embolic protection device. There were 10 (11.2%) subjects with MAEs reported during the 12-month follow-up period (in 89 patients), all of which were assessed by the reporting physicians as not directly related to the use of the 355 nm laser system. This included two (2.2%) Rutherford 5 subjects who had unplanned major target limb amputations, six (6.7%) subjects who underwent a clinically driven target lesion revascularization (TLR), and two (2.2%) subjects who underwent a target vessel revascularization. There were no cardiovascular deaths reported for the study population during the 12-month follow-up period and no death associated with the use of the 355 nm laser device.
4 DISCUSSION
The Pathfinder Registry represents the first prospective, large-scale multicenter real-world assessment of the 355 nm laser system in patients with PAD. This report is an evaluation of safety and efficacy at both 6 and 12 months, with continued patient follow-up planned out to 24 months.
The present analysis shows that the mean residual stenosis at the end of the index procedure was 24.4 ± 15.5. Further, nearly 70% of lesions treated in the registry had technical procedural success, defined as ≤30% residual stenosis at the index lesion postatherectomy and adjunctive therapy, despite 44.4% of lesions being CTOs and over a third of the subjects demonstrating long and/or moderate–severely calcified lesions. The rate of the residual stenosis could be attributed to the high-risk cohort with complex disease and a large percentage of critical limb ischemia (CLI). Figures 5-7 present fluoroscopy imaging of treated CTO, ISR, and severely calcified arteries (a) before the procedure, (b) following recanalization by the 355 nm laser, and (c) after balloon angioplasty with/without stenting. As seen in these figures, no signs of dissection, perforation or embolization were observed, neither following the laser application nor after the balloon angioplasty/stent.
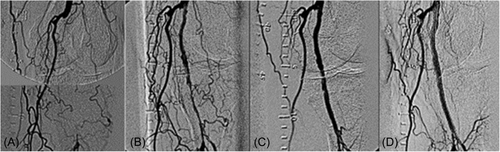
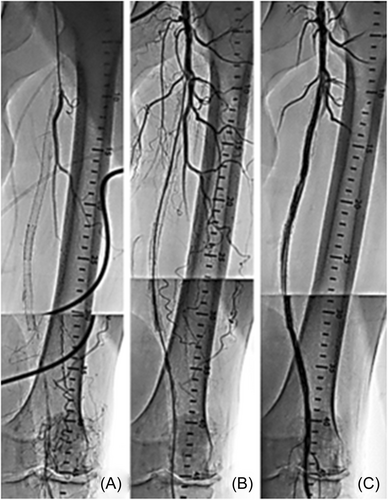
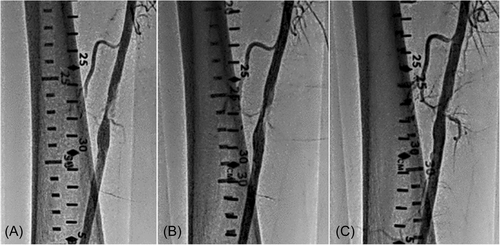
Clinical outcomes showed sustained improvement at both 6 and 12 months, with mean ABI/TBI, Rutherford classification, and WIQ overall scores showing significant improvement.
Overall, over 95% of patients achieved the primary safety endpoint of freedom from PPMAEs.
There was a low rate of adverse events with only 4.9% of subjects experiencing PPMAEs in an acute setting until discharge. Only one subject (1.0%) developed a clinically significant distal embolization as defined by the study protocol. No dissection was identified after the laser atherectomy alone. Two subjects (2.0%) required bailout stenting after balloon angioplasty and two (2.0%) subjects experienced postangioplasty grade C flow-limiting dissections that also required stents to be inserted. This overall low rate of dissection is a potential advantage of vessel preparation with the 355 nm laser before definitive angioplasty, and contrasts sharply in comparison to the >50% rate of dissections reported for BTK angioplasty alone.21 Noteworthy, there were no inpatient vessel perforations, amputations, or deaths until hospital discharge.
In the follow-up term, the 12-month rate of amputation in the Pathfinder cohort was relatively low (2.2%), especially in a population of over 40% CLI and BTK patients. Although these two major amputations were unplanned above the ankle for two CLI patients, the first amputation occurred 18 days postprocedure, was related to past medical history of severe PAD, and was deemed not related to the laser. The second amputation occurred 196 days postprocedure, was done below the knee in a CLI patient, and was also deemed not related to the laser. The 12-month rate of TLR was 6.7% (six of 89 subjects), which is also considered relatively low in this real-world patient population that included a high rate of patients with CLI, BTK, CTO, and ISR.
There are considerable technological advantages of the 355 nm laser over those of the longtime approved 308 nm laser, that is, significantly lower weight and size of the system, quiet mode of activation, no need for mandatory saline injections to dilute the contrast media, and less fluoroscopy dose.16 Moreover, the 355 nm laser has the inherent potential to modify plaque and thrombus,18 which could be of clinical importance due to the frequent presence of thrombus in patients with both acute and chronic presentations of peripheral arterial disease.22
The 355 nm laser System's design includes several unique features, which likely contribute to the procedural safety and efficacy observed in the Pathfinder Registry. The 355 nm laser's short-pulse duration between 10 and 25 ns allows a higher peak of energy over a shorter duration when compared to other laser technology. The combination of the unique 355 nm wavelength and the short pulse duration results in a much higher ablation affinity to the lesion material (plaque tissue) than the endothelial tissue or the deeper layers of the vessel wall.15, 16 The specificity of ablation to the plaque is enhanced by the calibrated and unique energy profile of the laser, which allows diffusion of generated heat and reduced thermal injury to the arterial wall as a result of a rapid repetition rate and extremely narrow pulse width. This mechanism of action is reflected in the absence of technical procedural complications in 95.1% of the patients. Furthermore, the short pulse duration allows the delivery of high laser power to disrupt severe medial calcium as seen in both the Investigational Device Exemption (IDE) EX-PAD-03 clinical study13, 14 and the recently reported ex vivo micro-CT study of 355 nm laser ablation in calcified tibial arteries.23
Although most of the demographic and clinical characteristics of the patient population for patients enrolled in the Pathfinder Registry were similar to those for the prospective, single-arm, multicenter US Food and Drug Administration-approved IDE clinical trial (NCT03157531) for the 355 nm laser atherectomy system, there were some notable differences for subjects enrolled in this real-world study.13, 14 The IDE trial only enrolled subjects from Rutherford Classification 2–4, whereas 24.1% of the subjects in the present study were Rutherford 5 and one patient was Rutherford 6. In addition to the relatively high rate of CLI patients (44.4% vs. 9.0%) and below the knee lesions (47.3% vs. 17.8%) in the present study versus the IDE trial, there were also twice as many subjects with chronic total occlusion in this real-world experience (44.4% vs. 21.5%), and a much higher rate of patients treated for restenosis/ISR (39.7% vs. 20.6%). Notably, drug-coated balloons were only used in 24.8% of the lesions compared to 51.4% of the lesions in the IDE EX-PAD-03 trial. From an outcome perspective, there was a 33.6% reduction in residual stenosis following use of the 355 nm laser device before adjunctive therapy for subjects in the IDE trial compared to a 26.4% reduction in the present study. The reduction observed for subjects in the Pathfinder Registry was independent of lesions characteristics. This is likely due to the difference in the patient and lesion characteristics with the subjects enrolled in the Pathfinder Registry having overall significantly more complex disease. During the follow-up period, subjects showed a positive trend of improved clinical outcomes at both 6 and 12 months, which is consistent with the results from the IDE study, despite the more complex disease treated.
There are several limitations associated with the present study. Although this was a prospective study, it did not include a control arm where alternative approaches or therapies were utilized during the same time interval. This limits the ability to compare the results observed in this study to other potential treatment strategies. There is also the potential for selection bias related to which patients were selected for procedures to be performed with the 355 nm laser system. Although subjects enrolled in the registry represent a heterogeneous population, patients subjectively excluded from being treated with the device could have influenced our findings. The difference in lesions count between the site reported and the Core Lab could be explained by the methodology of combining adjacent lesions (reported separately by the sites) for their angiographic analysis. In addition, intravascular ultrasound (IVUS) was not routinely used in this registry study (39.2%), and the consistent use of IVUS with optimized balloon sizing would have been expected to further improve acute results and potentially have an impact on early recoil.24
Further analysis of the Pathfinder Registry data will include the analysis of 24-month outcomes data, when available, and may include sub-group analyses based on the differing patient characteristics.
5 CONCLUSION
The Pathfinder Registry demonstrated the 355 nm laser System is both safe and effective for performing atherectomy procedures in patients with infrainguinal PAD in a real-world setting. Procedural results and clinical outcomes were similar across differing lesion types and characteristics with sustained clinical improvements at 6 and 12 months.
ACKNOWLEDGMENTS
We are grateful to the study site personnel and their patients for their support and involvement in this study. Larry Yost provided medical writing assistance from The Atticus Group, LLC (Portsmouth, New Hampshire, USA). We would also like to acknowledge the assistance of Oshrat Cohen and Ariel Bloch from AngioDynamics whose efforts to organize the registry, analyze the data and support for writing the manuscript were invaluable. Funding for the Pathfinder Registry, including data collection, monitoring, statistical analysis, and medical writing, was provided by AngioDynamics, Inc. (Latham, New York).
CONFLICT OF INTEREST STATEMENT
Nicolas W. Shammas, Jason A. Yoho, Pedro Martinez-Clark, VenkateshRamaiah, and John Rundback are consultants to AngioDynamics, Inc. (Latham, New York). None of the other authors has identified a conflict of interest related to the present work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.