Intra-stent aortic wall aneurysm formation after Be-graft covered stent implant
Abstract
Aortic wall injuries may occur after interventional treatment of aortic coarctation (CoA), especially after balloon angioplasty. We reported on a patient who presented with an intra-stent aneurysm formation after direct stenting of a native near atretic aortic CoA by using a BeGraft Aortic stent. This evidence supports the need to maintain a strict follow-up protocol. A computed tomography scan is mandatory, after covered stent implantation as well, especially in high-risk cases and even in the absence of any immediate apparent complication.
1 INTRODUCTION
Aortic wall injuries (AWIs) may occur after interventional treatment of aortic coarctation (CoA), especially after balloon angioplasty. The extended use of bare metal or covered stents diminished but not abolished the risk of these complication.
Here we reported on a patient who presented with an intra-stent aneurysm formation after direct stenting of a native near atretic aortic CoA by using a BeGraft Aortic stent (Bentley Innomed).
2 CASE REPORT
Aortic CoA associated with a bicuspid aortic valve was diagnosed in an 8-year-old boy with a body weight of 28.8 kg, presenting with a cardiac pathological systolic interscapular thrill and hypertension with upper/lower limbs pressure gradient of 40 mmHg at rest. A transthoracic echocardiogram confirmed the clinical suspicion of CoA, showing severe narrowing at the aortic isthmic region and high-speed blood flow (peak gradient of 40 mmHg) with diastolic runoff at continuous wave Doppler interrogation and severely reduced flow in the abdominal aorta.
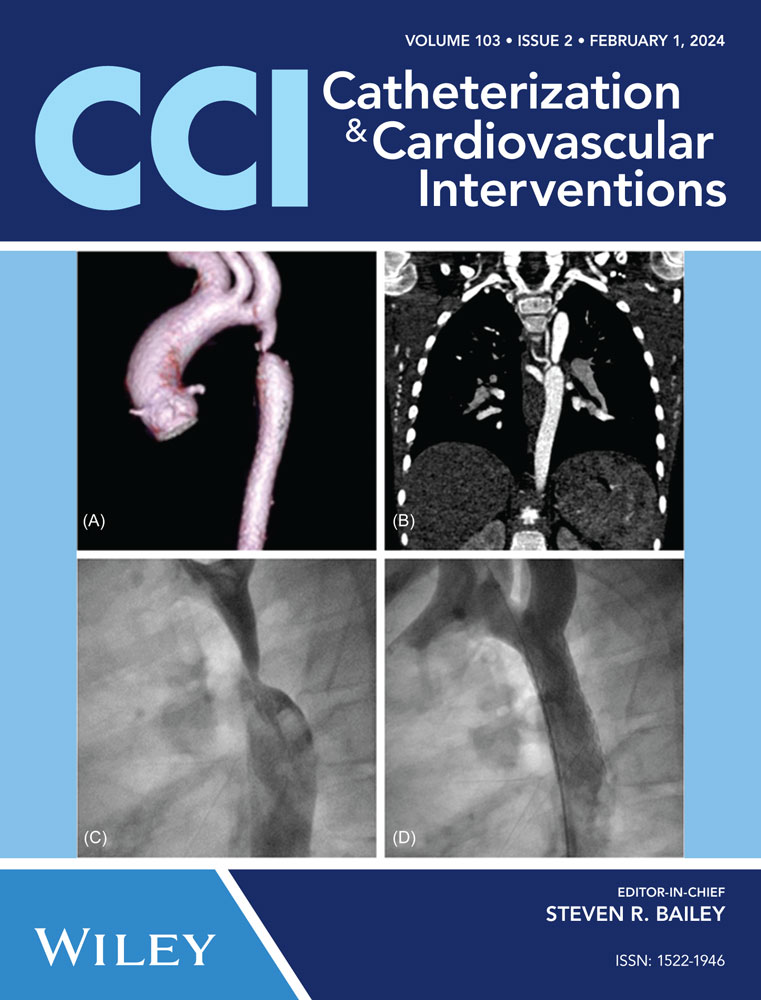
Angio computed tomography (CT) scan at diagnosis (Figure 1) showed a left aortic arch with a focal severe juxta ductal CoA at 18 mm from the left subclavian artery, with nearly atretic isthmus, post-CoA ectasia and normal diameters of the transverse arch and aorta at the diaphragm (11 × 12 mm and 12 × 12 mm, respectively) in the presence of multiple collateral vessels.
His case was discussed with the Heart team, even if a surgical option should be considered in similar cases, especially for the concern about future hypoplasia of the transverse arch and the need for re-intervention, in our case we opted for interventionism, and he underwent a cardiac catheterization short time after the diagnosis for CoA treatment. Percutaneous puncture of both femoral arteries with 6 Fr, 9 Fr, and humeral artery with 5Fr sheaths was performed. The hemodynamic assessment showed a peak-to-peak gradient of 40 mmHg across the aortic arch, under general anesthesia. Angiography confirmed a near subatretic isthmic CoA, mild post-stenotic aortic dilatation, and normal size at the transverse arch and descendent aorta at the diaphragm (12 × 12 mm). A 12 × 39 mm BeGraft stent was directly implanted retrogradely over a super stiff Amplatzer guidewire 0.35 inflating the balloon at 7 atm (which was within the recommended range), with an optimal angiographic result with no residual waist. The post-implant invasive peak-to-peak gradient was 5 mmHg. The patient was discharged the day after on acetylsalicylic acid and anti-hypertensive treatment in good clinical conditions, without upper/lower limbs systolic gradient.
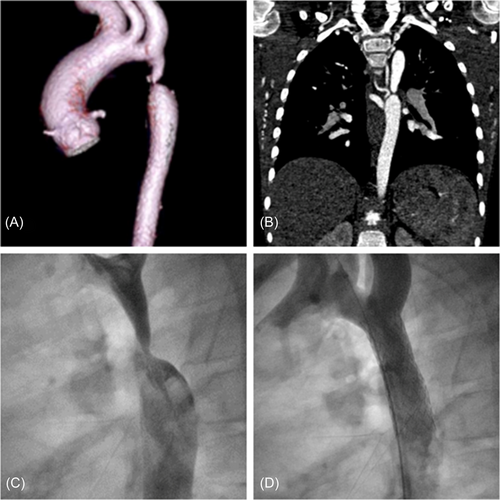
He remained in good clinical condition on a single antihypertensive drug. During follow-up, he developed poor control of arterial hypertension, a noninvasive blood pressure gradient of 20 mmHg, and an aortic arch systolic velocity on echo of 3 m/s with diastolic run-off. A CT scan was carried out 1 year later and revealed the formation of an aneurysm localized in the medial region of the stent and a disproportion between the stent and the native aorta probably related to the somatic growth of the patient. After medical team discussion and parent informed consent a cardiac catheterization was scheduled. The procedure was performed under general anesthesia and orotracheal intubation and arterial femoral access was obtained with sheaths up to 12 French. The residual peak-to-peak gradient was 13 mmHg and angiography confirmed the presence of aneurysmal dilatation of the aortic wall in correspondence with the medial portion of the struts of the stent (Figure 2A,B). Evidence of intra-stent peeling was also seen. As a further confirmation, a hand injection at the level of the stent by using a 6 Fr Multipurpose catheter clearly showed the direct relationship between the stent lumen and the aneurysmal dilatation in absence of any strut fracture or endoleaks. A covered 8zig cheatham platinum (CP) stent 45 mm mounted on a BiB balloon 14 × 45 mm over a stiff guidewire placed into the right subclavian artery was implanted in the target lesion to cover the aneurysmal segment and safely treat the residual stenosis. A postdilatation with the intent of flaring the stent with the aortic wall at his proximal and distal site was performed with a low-pressure balloon (Tyshak II 16 × 30 mm, NuMed). The postprocedure invasive gradient was 5 mmHg and the aneurysm was totally excluded (Figure 2B,C).
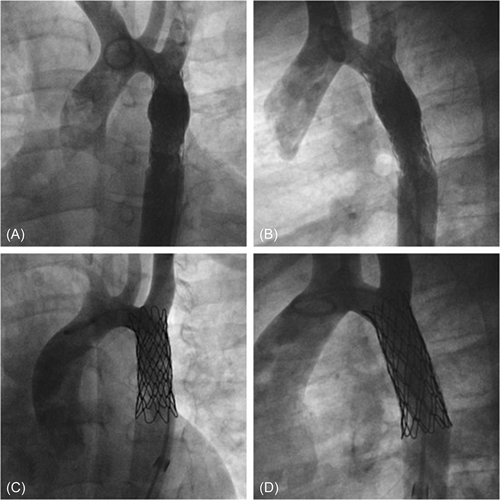
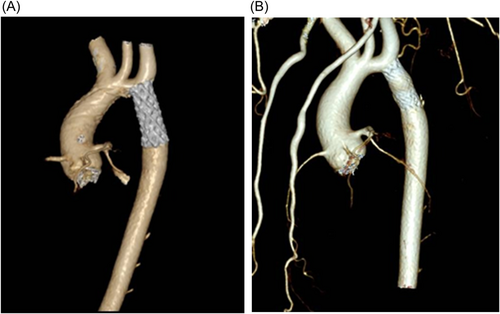
A follow-up CT scan was performed 6 months later showing no aneurysm formation and no other pathological images of the aortic wall (Figure 3A,B). On physical examination, he had good peripheral arterial pulses, no noninvasive BP cuff gradient between arms and legs, and on echo aortic arch systolic velocity was below 2 m/s. Furthermore, blood pressure was perfectly under control, and withdrawal of therapy is scheduled.
3 DISCUSSION
In the last three decades, percutaneous treatment of CoA has been widely used as an alternative treatment to surgery, with balloon angioplasty being the preferred technique in newborns and infants and stent placement in older children and adults. So far, the most frequently used covered stents have been the Cheatham platinum (NuMED, Inc.). More recently, the BeGraft aortic stent appeared as an appealing alternative, especially in smaller children (Bentley Innomed).1 The BeGraft is a premounted open cell stent graft made of a chromium-cobalt wire mesh covered by a polytetrafloroethylene (PTFE) tube. Is available in 12–24 mm diameters in 2 mm increments and lengths of 19–59 mm, and in vitro tests showed its capability of postdilation up to 30 mm. One of the main benefits relies on its relatively low profile and the possibility of delivery with smaller sheaths compared to covered CP stents (9 Fr for the 12 mm, 11 Fr for the 14–16 mm, and 14 Fr for 18–24 mm diameters respectively). Despite the overall decrease in the incidence of the majority of AWI (aneurysms, rupture, dissections) in the last few years, it remains one of the most concerning adverse events of aortic interventions.2 Recently the results of long-term follow-up after CoA stenting with covered CP stents (COAST II) have been published, showing that aneurysms can occur equally in patients after placement of bare metal or covered stents (COAST: 6/121, vs. COAST II: 7/127, 5.5%), and most aneurysms developed at the level of stent borders. A possible explanation can be that the pressure within the aorta could distribute the flow between the stent and the aortic wall, eventually leading to aneurysm formation mostly at the end of the stent at the interface with the aortic tissue mostly when the diseased tissue is not perfectly covered. Another possibility is that the expanded PTFE could develop damage during or after the implantation procedure.3 Covered stents are nowadays preferred for patients with very tight stenosis, or other characteristics that may act as risk factors for AWI occurrence (EJ. older age, genetic syndromes). Another interesting finding of the COAST trial is that only a minor percentage of aneurysms have been founded on MRI, being necessary in most cases, to perform a CT scan or a cardiac catheterization to make a diagnosis, and so the incidence of aneurysms at late follow-up could even be underestimated. In line with this evidence, CT scanning should be performed 1-year postimplantation and then every 2–5 years thereafter, depending on the patient's blood pressure and echocardiographic findings. Few studies are available on long-term results and complications of BeGraft stents in aortic CoA: there is only one report of an intra-stent aneurism in a patient, but it was related to a local distortion of a stent strut caused by unintentional pushing of the distal tip of the long sheath while withdrawing the balloon.4 In our patient, the final angiogram after stent implantation didn't show any immediate complications like small dissection or endoleaks, so probably the aneurism formation could be related to a small rupture of the PTFE coating that worsened over time. This was detected by an early CT scan that patients in our center perform during follow-up. Representatives of the Bentley company, who were directly contacted, are not aware of any occurrence of small tears in PTFE coating while expanding even at larger diameters than in our patient. Our decision was then to cover the aneurismatic formation with a CP-covered stent that extended some millimeters over the previous stent to cover completely the diseased tissue. The intended procedure was successful because the next tomographic scan showed a complete seal within the aortic wall and the stent struts with the total exclusion of the aneurysm.
4 CONCLUSION
To our best knowledge, this is the first report of a late aneurysm formation within the struts of a BeGraft Aortic stent. The main evidence provided from our experience highlights the need to maintain a strict follow-up protocol with CT scan surveillance on a short-term basis also after covered stent implantation, especially in high-risk cases and even in the absence of any immediate apparent complication.
ACKNOWLEDGMENTS
Open access funding provided by BIBLIOSAN.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.