Outcomes of non-flow-limiting spiral dissection after drug-coated balloon angioplasty for de novo femoropopliteal lesions
Abstract
Background
Whether drug-coated balloon (DCB) angioplasty would be effective in spiral dissection (SD) lesions with no flow impairment has been thoroughly investigated.
Aims
The present study sought to assess the clinical outcomes of non-flow-limiting SD after DCB angioplasty for de novo femoropopliteal lesions in patients with symptomatic lower extremity artery disease.
Method
This single-center retrospective study enrolled 497 patients with non-flow-limiting SD (n = 92) or non-SD (n = 405) without bailout stenting. The primary endpoint was 1-year primary patency, with the secondary endpoints including freedom from target lesion revascularization (TLR), major adverse limb event (MALE), all-cause death, and 30-day restenosis.
Results
The 1-year primary patency and freedom from TLR were significantly lower in the SD group than in the non-SD group (69.8% vs. 83.3%, p = 0.004; 78.7% vs. 93.0%, p = 0.007, respectively). The SD group had a higher incidence of MALE and 30-day restenosis than the non-SD group (24.6% vs. 11.9%, p = 0.001; 4.3% vs. 0.5%, p = 0.002, respectively). All-cause death was comparable. One-year restenosis after SD was associated with chronic limb-threatening ischemia (CLTI) (hazard ratio, 3.36 [95% confidence interval, 1.21–9.36]; p = 0.020), TASC Ⅱ D (hazard ratio, 3.97 [95% confidence interval, 1.02–15.52]; p = 0.047), and residual stenosis ≥50% (hazard ratio, 4.92 [95% confidence interval, 1.01–23.94]; p = 0.048). The incidence of restenosis after SD increased with the number of these risk factors.
Conclusions
Despite normal antegrade flow, the 1-year primary patency rate after DCB angioplasty for de novo femoropopliteal lesions was significantly lower in lesions with SD than those without SD. CLTI, TASC II D, and residual stenosis ≥50% were risk factors associated with 1-year restenosis after DCB angioplasty for non-flow-limiting SD lesions.
Abbreviations
-
- CLTI
-
- chronic limb-threatening ischemia
-
- DCB
-
- drug-coated balloon
-
- EVT
-
- endovascular treatment
-
- IVUS
-
- intravascular ultrasound
-
- MALE
-
- major adverse limb event
-
- PACSS
-
- Peripheral Artery Calcification Scoring System
-
- SD
-
- spiral dissection
-
- TASC
-
- Trans-Atlantic Inter-Society Consensus
-
- TLR
-
- target lesion revascularization
1 BACKGROUND
Current guidelines recommend drug-coated balloon (DCB) angioplasty as the first-line endovascular treatment (EVT) for femoropopliteal lesions.1-3 However, dissection remains an unsolved issue after balloon angioplasty, which might lead to deleterious clinical consequences (e.g., acute vessel occlusion and early restenosis), particularly in cases of severe dissection.4 Classifying angioplasty-induced femoropopliteal dissection can help decision-making for EVT strategy (DCB or scaffolding), while there was no dedicated and comprehensive system for those dissections. Recently, the DISFORM classification system has been developed as a novel grading and treatment algorithm for femoropopliteal artery dissection; it consists of four critical features, including (1) diameter reduction, (2) spiral shape, (3) flow impairment, and (4) adverse morphology.5 This system provides a clear, concise decision aid to individual interventionists facing any dissections in clinical practice.
Spiral dissection (SD) is a higher-grade dissection and requires scaffolding with stents or tacks when antegrade flow decreases (FLIPI [flow limitation in peripheral interventions] ≥1).5 Meanwhile, some SD cases do not restrict antegrade flow, potentially being managed without bailout scaffolding.5 However, little data on outcomes after DCB angioplasty in femoropopliteal lesions with SD is available. The present study aimed to investigate the clinical outcomes of non-flow-limiting SD after DCB angioplasty for de novo femoropopliteal lesions.
2 METHODS
2.1 Study design and patient population
This was a retrospective observational study at Sapporo Heart Center, Sapporo Cardio Vascular Clinic, Sapporo, Japan. From June 2018 to December 2022, 842 consecutive patients with symptomatic lower extremity artery disease underwent EVT using DCBs. In the present study, we retrospectively sought to enroll patients who had de novo femoropopliteal lesions with a Rutherford classification ranging from 2 to 6.6 Patients with (1) flow-limiting dissections, (2) bailout scaffolding, (3) common femoral artery lesions, and (4) restenotic lesions were excluded from this study. The patients were divided into two groups according to the presence or absence of SD after DCB angioplasty. The study protocol was approved by the ethics committee at Sapporo Heart Center (No. 20230003) and was in accordance with the Declaration of Helsinki. Written informed consent was waived because of the retrospective study design.
2.2 Endovascular procedures and medical therapy
Patients with symptomatic femoropopliteal lesions exhibiting >70% diameter stenosis and a Rutherford classification between 2 and 6 were selected for EVT. A 6-Fr sheath was inserted into the common femoral artery according to lesion location, and a 5000-IU dose of unfractionated heparin was administered to achieve an activated coagulation time >250 s. Lesion crossing was accomplished using a 0.014- or 0.018-inch guidewire, with balloon selection based on lesion morphology and location. Balloon diameter was determined using angiography or intravascular ultrasound (IVUS), and DCB angioplasty was performed to cover the entire lesion. The choice of DCBs, either low-dose DCB (Lutonix, Becton Dickinson and Ranger, Boston Scientific) or high-dose DCB (IN.PACT Admiral, Medtronic), and specialty balloons (Cutting Balloon, Boston Scientific and AngioSculpt, Philips) was determined at the operator's discretion based on lesion severity such as a long lesion, small vessel, occlusion, and calcification. If residual stenosis after DCB angioplasty exceeded 50%, the lesion was evaluated to determine whether additional treatment was indicated based on pressure gradient or IVUS evaluation. Lesions determined to require additional treatment were post-dilated with an appropriately sized balloon based on angiographic or IVUS information. Atherectomy devices were not available during the study period. Hemostasis was achieved with manual compression or device closure. After DCB angioplasty, dual antiplatelet therapy (aspirin 100 mg daily and 75 mg clopidogrel daily) was recommended for 3 months. In patients receiving anticoagulant therapy, the combination of anticoagulant and dual antiplatelet therapy was recommended for 1 month, followed by long-term anticoagulation and aspirin therapy.
2.3 Study endpoints and follow-up
The primary endpoint was 1-year primary patency, defined as target lesion patency without significant restenosis. Secondary endpoints included early restenosis and 1-year clinical events (target lesion revascularization [TLR], major adverse limb event [MALE], and all-cause death). Restenosis was defined as a peak systolic velocity ratio >2.4 on duplex ultrasonography or >50% stenosis on follow-up computed tomography angiogram.7, 8 Early restenosis and major amputation were defined as occurring within 30 days after the index procedure. TLR was defined as a repeat EVT or surgical bypass procedure for limbs with recurrent symptoms and restenosis. MALE was defined as a composite of major amputation or any reintervention.9
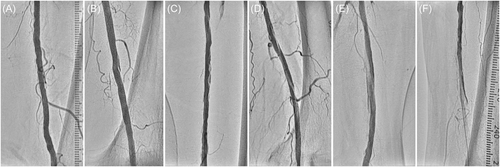
Patients were routinely followed up at our outpatient clinic at 1, 6, and 12 months or in the occurrence of clinical deterioration. The follow-up protocol consisted of postoperative ankle-brachial index and lower limb duplex ultrasound at each visit. If ultrasound results were inconclusive, computed tomography angiogram, digital subtraction angiography, or both were performed. The severity of dissection was graded according to the National Heart, Lung and Blood Institute classification system.10 Type A was defined as dissection with minor radiolucent areas, type B as linear dissection, type C as dissection with contrast outside the lumen, type D as spiral dissection, type E as persistent filling defects, and type F as total occlusion without distal antegrade flow (Figure 1). Flow-limiting dissection was defined as dissection with deterioration of the distal antegrade flow (flow-limiting type D, E, and F dissection). The severity of lower extremity artery disease was categorized according to the Rutherford classification as mild to severe intermittent claudication (class 1–3) and chronic limb-threatening ischemia (CLTI) with and without tissue loss (class 4–6).6 Lesion severity was graded according to the Trans-Atlantic Inter-Society Consensus (TASC) II classification.11 Calcification laterality was determined according to the Peripheral Artery Calcification Scoring System (PACSS) classification.12
2.4 Statistical analysis
Continuous variables were presented as mean ± SD and compared by the unpaired t-test or the Mann–Whitney U test. Categorical variables were expressed as numbers (percentages) and compared by the chi-square test. The cumulative 1-year incidence of study endpoints was estimated by the Kaplan–Meier method and compared by the log-rank test. We included 11 variables clinically relevant covariates for 1-year patency by the Cox models (below-the-knee runoff ≤1, CLTI, small vessel ≤4.0 mm, prior heart failure, hemodialysis, PACSS grade 4, residual stenosis ≥50%, IVUS use, low-dose DCB use, specialty balloon use, and TASC II D).3, 4, 12 All statistical analyses were performed by two physicians (T. H. and S. K.) using SPSS statistics version 29.0 (SPSS, Inc.) and R software version 3.5.2 (R Foundation for Statistical Computing). A two-sided p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Study population
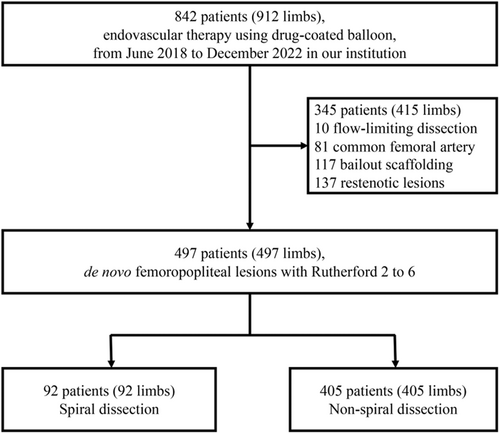
Among 842 patients (912 limbs), 345 were excluded due to flow-limiting dissections (n = 10), bailout scaffolding (n = 117), common femoral artery lesions (n = 81), and restenotic lesions (n = 137). Finally, 497 patients (497 limbs) were enrolled in the present study; non-flow-limiting SD was observed in 92 patients (92 limbs) (18.5%) (Figure 2).
3.2 Baseline clinical characteristics
Baseline patient and lesion characteristics are summarized in Table 1. There were no significant differences between the SD and non-SD groups. In terms of lesion characteristics, the SD group had considerably greater lesion complexity. Table 2 presents procedural characteristics and results between the two groups. Compared with the non-SD group, the SD group showed a smaller vessel diameter and a longer lesion length with less frequency of specialty balloon use, resulting in a smaller DCB size and a longer total DCB length. After DCB angioplasty, the SD group had a higher residual restenosis rate and a lower ankle-brachial index than the non-SD groups (15.3 ± 1.8% vs. 9.6 ± 0.7, p = 0.002; 0.90 ± 0.19 vs. 0.97 ± 0.18, p < 0.001, respectively). Periprocedural complications did not differ between groups.
| SD (n = 92) | Non-SD (n = 405) | p Value | |
|---|---|---|---|
| Age, years | 75.8 ± 8.7 | 77.7 ± 9.2 | 0.099 |
| Male: sex | 50 (54%) | 244 (60%) | 0.252 |
| Ambulatory | 62 (67%) | 290 (72%) | 0.328 |
| Diabetes mellitus | 46 (50%) | 206 (51%) | 0.740 |
| Hemodialysis | 15 (16%) | 79 (20%) | 0.417 |
| Statin | 48 (52%) | 197 (49%) | 0.605 |
| Chronic limb-threatening ischemia | 30 (32%) | 133 (33%) | 0.855 |
| Preoperative ankle brachial index | 0.58 ± 0.31 | 0.66 ± 0.28 | 0.078 |
| Distal reference vessel diameter, mm | 4.2 ± 1.1 | 4.7 ± 1.0 | <0.001 |
| Lesion length, mm | 263.9 ± 85.1 | 169.9 ± 104.5 | <0.001 |
| Preoperative stenosis, % | 95.1 ± 7.8 | 92.0 ± 9.2 | 0.003 |
| Chronic total occlusion | 43 (47%) | 108 (27%) | <0.001 |
| Occlusion length (mm) | 197.6 ± 118.8 | 111.8 ± 92.0 | <0.001 |
| TASC Ⅱ (A/B/C/D) | 3/8/60/21 | 120/73/191/21 | <0.001 |
| PACSS grade (1/2/3/4) | 6/15/7/35 | 41/36/50/143 | 0.068 |
| Below-the-knee poor-runoff ≤1 | 34 (37%) | 140 (35%) | 0.752 |
- Note: Data are presented as numbers (percentages) or means ± standard deviation unless otherwise specified.
- Abbreviations: PACSS, Peripheral Artery Calcium Scoring System; TASC, Trans-Atlantic Inter-Society Consensus.
| SD (n = 92) | Non-SD (n = 405) | p Value | |
|---|---|---|---|
| Vessel preparation | |||
| Diameter, mm | 4.9 ± 0.7 | 5.3 ± 0.6 | <0.001 |
| Diameter to artery ratio | 1.35 ± 0.39 | 1.26 ± 0.36 | 0.081 |
| Balloon length (mm) | 291.6 ± 170.3 | 179.9 ± 134.4 | <0.001 |
| Inflation pressure, atm | 16.6 ± 5.9 | 16.3 ± 7.1 | 0.754 |
| Inflation time (s) | 71.7 ± 138.1 | 50.3 ± 127.2 | 0.308 |
| Specialty balloon use | 16 (17%) | 158 (40%) | 0.002 |
| Drug-coated balloon | |||
| Number of balloons used | 1.6 ± 0.5 | 1.3 ± 0.5 | <0.001 |
| Low-dose drug-coated balloons | 67 (73%) | 270 (67%) | 0.317 |
| Diameter (mm) | 5.4 ± 0.7 | 5.6 ± 0.6 | 0.007 |
| Balloon/artery diameter | 1.36 ± 0.39 | 1.25 ± 0.34 | 0.007 |
| Length (mm) | 274.5 ± 103.2 | 185.4 ± 108.3 | <0.001 |
| Inflation pressure (atm) | 13.4 ± 2.2 | 12.7 ± 2.8 | 0.108 |
| Inflation time (s) | 175.4 ± 65.0 | 163.0 ± 67.0 | 0.235 |
| Intravascular ultrasound use | 36 (39%) | 102 (25%) | 0.009 |
| Dissection classification (none/A/B/C) | - | 60/77/159/109 | - |
| At the index procedure | |||
| Postoperative ankle brachial index | 0.90 ± 0.19 | 0.97 ± 0.18 | <0.001 |
| Postoperative stenosis, % | 15.3 ± 1.8 | 9.6 ± 0.7 | 0.002 |
| Residual stenosis <30% | 85 (91%) | 392 (97%) | 0.027 |
| Periprocedural complications | 3 (3.2%) | 9 (2.2%) | 0.443 |
- Note: Data are presented as numbers (percentages) or means ± standard deviation unless otherwise specified.
3.3 Impact of SD on clinical outcomes
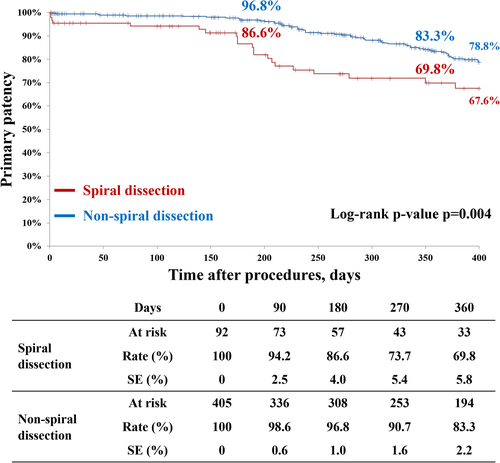
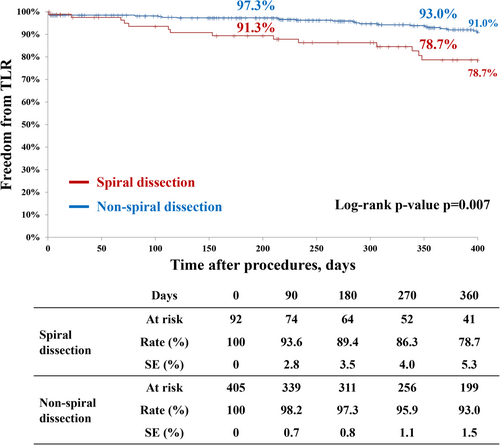
Table 3 demonstrates clinical outcomes after DCB angioplasty in the SD and non-SD groups. The 1-year primary patency and freedom from TLR were significantly lower in the SD group than in the non-SD group (69.8% vs. 83.3%, p = 0.004; 78.7% vs. 93.0%, p = 0.007, respectively) (Figures 3 and 4). Similarly, early restenosis and 1-year clinical events (MALE and major amputation) occurred more frequently in the SD group than in the non-SD group (4.3% vs. 0.5%, p = 0.002; 24.6% vs. 11.9%, p = 0.001; 9.6% vs. 1.7%, p = 0.001, respectively). All-cause death and early major amputation were comparable in both groups.
| SD (n = 92) | Non-SD (n = 405) | p Value | |
|---|---|---|---|
| At 1 year | |||
| Primary patency | 69.8% (71) | 83.3% (345) | 0.004 |
| Freedom from target lesion revascularization | 78.7% (76) | 93.0% (370) | 0.007 |
| Major adverse limb event | 24.6% (19) | 11.9% (41) | 0.001 |
| Major amputation | 9.6% (7) | 1.7% (6) | 0.001 |
| All-cause death | 22.6% (16) | 16.7% (57) | 0.343 |
| At 30 days | |||
| Restenosisa | 4.3% (4) | 0.5% (2) | 0.002 |
| Major amputation | 1.1% (1) | 0.2% (1) | 0.256 |
- Note: Values are presented as Kaplan–Meier estimates (number of events).
- a The median duration from the index procedure was 1.7 and 3.5 days in the SD and non-SD groups, respectively.
3.4 Factors associated with restenosis after SD
CLTI (hazard ratio, 3.36 [95% confidence interval, 1.21–9.36]; p = 0.020), TASC Ⅱ D (hazard ratio, 3.97 [95% confidence interval, 1.02–15.52]; p = 0.047), and residual stenosis ≥50% (hazard ratio, 4.92 [95% confidence interval, 1.01–23.94]; p = 0.048) were associated with 1-year restenosis after SD occurrence (Table 4).
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p Value | Hazard ratio (95% CI) | p Value | |
| Prior heart failure | 2.23 (0.81–6.10) | 0.121 | 1.61 (0.48–5.33) | 0.439 |
| Hemodialysis | 1.31 (0.38–4.46) | 0.668 | 0.79 (0.17–3.67) | 0.761 |
| Chronic limb threatening ischemia | 3.76 (1.55–9.13) | 0.003 | 3.36 (1.21–9.36) | 0.020 |
| Distal reference vessel diameter ≤4.0 mm | 2.15 (0.91–5.11) | 0.082 | 2.56 (0.79–8.27) | 0.117 |
| TASC Ⅱ D | 3.58 (1.50–8.53) | 0.004 | 3.97 (1.02–15.52) | 0.047 |
| PACSS grade 4 | 1.52 (0.65–3.59) | 0.336 | 2.66 (0.84–8.41) | 0.095 |
| Below-the-knee runoff ≤1 | 1.44 (0.61–3.40) | 0.399 | 0.64 (0.21–1.92) | 0.424 |
| Residual stenosis ≥50% | 2.28 (0.67–7.76) | 0.188 | 4.92 (1.01–23.94) | 0.048 |
| Low-dose DCB | 1.67 (0.61–4.60) | 0.319 | 0.90 (0.26–3.12) | 0.863 |
| IVUS use | 1.58 (0.67–3.73) | 0.294 | 0.57 (0.14–2.26) | 0.422 |
| Specialty balloon use | 0.36 (0.08–1.54) | 0.169 | 0.29 (0.05–1.70) | 0.170 |
- Abbreviations: CI, confidence interval; DCB, drug-coated balloon; IVUS, intravascular ultrasound; TASC, Trans-Atlantic Inter-Society Consensus; PACSS, Peripheral Artery Calcification Scoring System.
4 DISCUSSION
The main findings of the present study can be summarized as follows: (1) 1-year primary patency and freedom from TLR were significantly lower in the SD group than in the non-SD group; (2) early restenosis and MALE occurred more frequently in the SD group compared to the non-SD group; (3) CLTI, TASC II D, and residual stenosis ≥50% were risk factors of 1-year restenosis after DCB angioplasty for femoropopliteal lesions with non-flow-limiting SD; and (4) the 1-year primary patency decreased as the number of these risk factors (i.e., CLTI, TASC II D, and residual stenosis ≥50%) increased.
DCB angioplasty emerges as the first-line EVT for femoropopliteal lesions in contemporary practice.1-3 Although optimal lesion preparation before DCB angioplasty is crucial to obtain better outcomes,1-4 dissection occurrence is unavoidable due to the underlying mechanism of balloon angioplasty. Since severe dissection might result in deleterious clinical consequences, dissection classification is of most importance to decision-making for EVT strategy (scaffolding or DCB). The current expert consensus strongly recommends scaffold implantation for any dissections having at least one of the following features: (1) diameter reduction ≥50% of the lumen, (2) SD, and (3) severe flow reduction (FLIPI 2 or 3).5 Also, scaffolding is moderately recommended for SD with no flow impairment. However, little evidence is available regarding the clinical outcomes of DCB angioplasty for de novo femoropopliteal lesions with non-flow-limiting SD. To our knowledge, the present study is the first to investigate whether DCB angioplasty would be effective for de novo femoropopliteal lesions with non-flow-limiting SD. In the present study, 1-year primary patency and freedom from TLR were significantly lower in the SD group than in the non-SD group; early restenosis and 1-year MALE and major amputation occurred more frequently in the SD group than in the non-SD group. Our results support the scaffolding usage for non-flow-limiting SD lesions, filling a gap between the expert consensus recommendation and daily practice.
Recently, a large-scale prospective multicenter registry reported that risk factors associated with 1-year restenosis after DCB angioplasty for femoropopliteal lesions included a history of EVT, smaller distal reference vessel diameter, severe calcification, chronic total occlusion, low-dose DCB, and residual restenosis.3 To date, however, no clinical study assessed the risk factors for 1-year restenosis of non-flow-limiting SD after DCB angioplasty for femoropopliteal lesions. The present study demonstrated that CLTI, TASC II D, and residual stenosis ≥50% were risk factors for 1-year restenosis after DCB angioplasty for SD lesions, which was not in line with the previous study except for residual stenosis.3 Possible explanations for this include the following: (1) the incidence of SD was lower in the previous study than in the present study (3.9% vs. 18.5%).3 This may be because the balloons used in this study, including the DCB, had a balloon-to-artery ratio of 1.36 in the SD group and 1.25 in the non-SD group, which is larger than the generally recommended 1:1 ratio; (2) SD lesions have a more severe lesion nature associated with worse outcomes after DCB angioplasty. Due to the complex clinical backgrounds, CLTI patients have a poor limb and life prognosis, and their lesion severity might be unquantifiable by angiography.6, 11-13 Moreover, the TASC II D lesion and residual stenosis are well-established restenosis factors after EVT.3, 11, 12 In addition, the different doses of paclitaxel were not associated with restenosis after the occurrence of SD. Although the clinical benefit of DCB angioplasty has been limited in patients with CLTI, TASC II D lesions, and residual stenosis ≥50%, the present study suggested that its efficacy might be further attenuated in those with SD.
In the current study, the 1-year primary patency decreased as the number of risk factors associated with 1-year restenosis (i.e., CLTI, TASC II D, and residual stenosis ≥50%) increased. It is noteworthy that the 1-year patency rate after DCB angioplasty (80.6%) seemed clinically acceptable in SD cases without any risk factors. In contrast, the patency rate remarkably worsened when the risk factor was present (1 risk factor, 61.8%; 2 risk factors, 48.9%; 3 risk factors, 0%), indicating the need for scaffold implantation instead of DCB angioplasty. Consistently, Soga et al. reported (1) the 1-year patency rate after DCB angioplasty for femoropopliteal lesions reduced in patients with multiple restenosis risk factors and (2) the 1-year patency rate appeared clinically acceptable in patients with ≤2 risk factors (1 risk factor, 85.8%; 2 risk factors, 78.5%) but not in those with ≥3 risk factors (3 risk factors, 65.8%; >3 risk factors, 58.6%).3 Given these findings, assessing the number of restenosis risk factors might help decide the appropriate EVT strategy (DCB or scaffolds) for non-flow-limiting SD lesions.
4.1 Limitations
The present study has several limitations. First, the present study was a retrospective study at a single center. Furthermore, lesion characteristics and angiographic images were not analyzed in an independent core laboratory. As such, these findings might have biased the present study results and conclusions. Second, compared with two-dimensional angiographic imaging, IVUS is a more accurate tool for detecting the presence of dissection. However, the current study performed an IVUS examination in 27.8% of all cases, which might result in underestimating SD incidence. Third, atherectomy devices were not used in the present study since they were unavailable during the study period. Fourth, given that the present study excluded cases with bailout stenting for dissection, further studies are warranted to compare the clinical outcomes between DCB and drug-eluting stents in non-flow-limiting SD. Fifth, we did not obtain information on the pressure gradient within the lesions in the present study. A combined assessment of dissection using angiographic images and pressure gradients might have provided more comprehensive information on the EVT strategy. Sixth, as the SD group was relatively small, our results should be considered hypothesis-generating. An optimal strategy for non-flow-limiting SD should be addressed shortly. Finally, extrapolating our results outside Japan requires caution because the study population consisted solely of Japanese subjects.
5 CONCLUSIONS
Despite normal antegrade flow, the 1-year primary patency rate after DCB angioplasty for de novo femoropopliteal lesions was significantly lower in lesions with SD than those without SD. CLTI, TASC II D, and residual stenosis ≥50% were risk factors associated with 1-year restenosis after DCB angioplasty for non-flow-limiting SD lesions. Notably, the 1-year patency rate seemed clinically acceptable when SD lesions had no such risk factors, although it remarkably worsened with them.
ACKNOWLEDGMENTS
The authors thank all the staff of the Sapporo Heart Center.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data associated with this research work are available from the corresponding author upon reasonable request.