Impact of balloon post-dilation on valve durability and long-term clinical outcomes after self-expanding transcatheter aortic valve implantation
Jorge Sanz Sánchez and Damiano Regazzoli contributed equally to this work.
Abstract
Background
Balloon post-dilation (BPD) is a widely adopted strategy to optimize acute results of TAVI, with a positive impact on both paravalvular leak and mean gradients. On the other hand, the inflation of the balloon inside prosthetic leaflets may damage them increasing the risk of structural valve deterioration (SVD). Furthermore, the impact of BPD on long-term clinical outcomes and valve hemodynamics is yet unknown.
Aims
To evaluate the impact of BPD on valve durability and long-term clinical outcomes in patients undergoing self-expanding transcatheter valve implantation (TAVI).
Methods
Echocardiographic and clinical data from the ClinicalService (a nation-based data repository and medical care project) were analyzed. Patients were divided into two groups, those who underwent BPD after TAVI and those who did not. Coprimary endpoints were all-cause death and SVD. Cumulative incidence functions for SVD were estimated.
Results
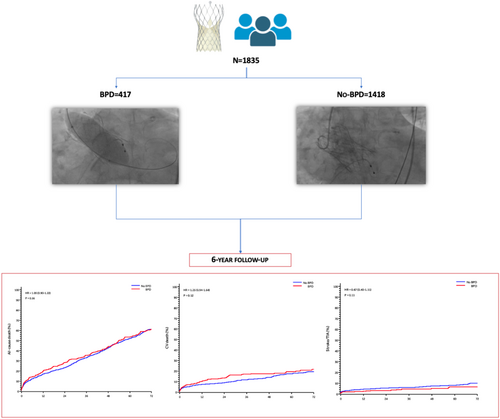
Among 1835 patients included in the study, 417 (22.7%) underwent BPD and 1418 (77.3%) did not undergo BPD. No statistically significant differences at 6-year follow-up were found between groups in terms of all-cause mortality (HR: 1.05, 95% CI: 0.9–1.22; p = 0.557) and SVD (2.1% vs. 1.4%, p = 0.381). In addition, BPD did not predispose to higher risk of cardiovascular death, myocardial infarction, valve thrombosis, and endocarditis at 6-year follow-up.
Conclusions
BPD following TAVI with a self-expanding prosthesis does not seem to be associated with an increased risk of adverse clinical outcomes or SVD at 6-year follow-up.
Abbreviations
-
- BPD
-
- balloon post-dilation
-
- CI
-
- confidence interval
-
- CIF
-
- cumulative incidence function
-
- HR
-
- hazard ratio
-
- PVL
-
- paravalvular leak
-
- SVD
-
- structural valve deterioration
-
- TAVI
-
- transcatheter valve implantation
1 INTRODUCTION
Transcatheter aortic valve implantation (TAVI) is nowadays the first line therapy for the treatment of aortic valve stenosis in patients at high, intermediate, and low surgical risk.1-3 Moderate paravalvular leak (PVL) after TAVI is, since early generation devices, a well-known complication occurring in up to 22% of the cases and has been associated with worse clinical outcomes and increased long-term mortality.4-6 Balloon post-dilation (BPD) is a widely adopted strategy to reduce the degree of PVL after TAVI in case of prosthesis under-expansion.7 Furthermore, BPD may play a major role also in optimizing valve expansion and therefore may lead to decreased risk of prosthesis-patient mismatch.8 However, it might be associated with potential damage of the prosthetic leaflets leading to more rapid deterioration and structural failure of the transcatheter valve.9 In addition, initial single center observations with limited populations warned that BPD might be associated with an increased risk of iatrogenic cerebrovascular events.7, 10 However, latter larger multicentric observational studies did not confirm these results and showed that BPD after TAVI was safe and not associated with increased risk of stroke, aortic root injury, and mortality.11 Nevertheless, follow-up duration of the aforementioned studies is limited (12 months), and the impact of BPD on long-term valve hemodynamics after TAVI remains unclear. Therefore, the aim of this large multicenter observational study was to evaluate the impact of BPD on valve durability and long-term clinical outcomes after TAVI with a self-expanding prosthesis.
2 METHODS
2.1 Patient population
The study design of the Clinical Service Project has been previously described.12 Briefly, between June 2007 and December 2014 all consecutive patients with severe aortic stenosis undergoing TAVI with the CoreValve and Evolut R (Medtronic Inc.) devices in eight Italian centers were prospectively included. Clinical Service Project is a nation-based clinical data repository and medical care quality improvement project aimed at describing and improving the use of implantable devices in Italian clinical practice (clinicaltrials.gov NCT01007474). The project was approved by each site's institutional review board or medical director and conforms to the principles outlined in the Declaration of Helsinki. Each patient signed an informed consent for data collection and analysis. Clinical and echocardiographic follow-up were performed according to each center's clinical practice. All events were site reported and centrally readjudicated.
The current substudy aimed to evaluate the impact of BPD on valve durability and long-term clinical outcomes after TAVI with the CoreValve and Evolut R prosthesis. Patients were divided into two groups, those who underwent BPD after TAVI and those who did not undergo BPD.
2.2 Endpoints and definitions
Coprimary endpoints were all-cause death and structural valve deterioration (SVD) at longest available follow-up. SVD was defined according to VARC-3 criteria.13 Secondary endpoints included cardiovascular death, myocardial infarction, stroke or transient ischemic attack, new pacemaker implantation, aortic surgery, valve thrombosis, and endocarditis. In addition, 6-year follow-up echocardiographic data is provided.
Detailed outcome definitions and clinical events committee procedures have been published elsewhere. 12
2.3 Statistical analysis
Continuous variables are reported as mean ± standard deviation or median ± interquartile range and were compared using Student's t-test or the Mann−Whitney U or Wilcoxon test in case of two-group comparisons on the basis of normality of data distribution, verified using the Shapiro−Wilk test. For categorical variables, absolute and relative frequencies are reported. Differences between groups were evaluated using χ2 test or Fisher's exact test, as appropriate.
The analyses of time-to-first event were described using Kaplan−Meier curves and compared between groups with the log-rank test. Cox regressions were performed for both univariable and multivariable analyses, and the proportional hazard hypothesis was tested. Hazard ratios (HRs) were reported together with 95% confidence intervals. Unless noted otherwise, statistically significant parameters in univariable analysis (p < 0.10) were analyzed in multivariable analysis with the appropriate selection, and stepwise selection with entry criteria = 0.30, stay criteria = 0.05. Collinearity diagnostic was examined by correlation between covariates included into the multivariable model, through variance inflation factor and tolerance, and in terms of proportion of variation of covariates.
Hemodynamic SVD rates were estimated using the cumulative incidence function (CIF) with all-cause death as the competing risk for hemodynamic SVD, as appropriate. The CIF were compared between groups using Gray's test.
A two-sided p < 0.05 was considered statistically significant. Statistical analyses were performed using Stata version 13.0 (StataCorp.).
3 RESULTS
3.1 Study population and clinical features
A total of 1835 patients undergoing TAVI were included in the analysis. Of these, 417 (22.7%) underwent BPD and 1418 (77.3%) did not undergo BPD. Table 1 reports clinical and echocardiographic characteristics stratified according to the performance of BPD. Patients undergoing BPD as compared to those not undergoing BPD were more frequently male and had higher rates of atrial fibrillation and New York Heart Association functional class III or IV at clinical presentation. Conversely, previous percutaneous coronary intervention was more frequent among no-BPD patients. Baseline echocardiography showed that patients undergoing BPD had higher mean aortic gradients at baseline and a higher rate of severe mitral and aortic regurgitation.
| Overall (N = 1835) | BPD (N = 417) | No-BPD (N = 1418) | p Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) | 82 ± 6 | 81 ± 7 | 82 ± 6 | 0.07 |
| Male | 830 (45.2) | 633 (72) | 236 (56.6) | <0.001 |
| BMI (kg/m)2 | 26 ± 5 | 26 ± 5 | 26 ± 5 | 0.853 |
| Hypertension | 1484 (81.1) | 338 (81.1) | 1146 (81.1) | 0.982 |
| Diabetes mellitus | 551 (30.3) | 113 (27.3) | 438 (31.2) | 0.129 |
| GFR ≤ 30 mL/min | 389 (21.6) | 91 (22.1) | 298 (21.4) | 0.768 |
| Peripheral vascular disease | 496 (27) | 124 (29.7) | 372 (26.2) | 0.157 |
| Severe COPD | 402 (21.9) | 84 (20.1) | 318 (22.4) | 0.322 |
| NYHA III or IV | 1361 (76.7) | 332 (82.4) | 1029 (75) | 0.002 |
| Atrial fibrillation | 379 (20.7) | 102 (24.5) | 277 (19.5) | 0.029 |
| STS score (%) | 6.0 (3.9−9.9) | 6.4 (3.9−10.4) | 5.9 (3.9−9.7) | 0.525 |
| LBBB | 187 (10.2) | 32 (7.7) | 155 (10.9) | 0.053 |
| RBBB | 129 (7) | 33 (7.9) | 96 (6.8) | 0.422 |
| Previous MI | 326 (18.1) | 78 (19) | 248 (17.9) | 0.597 |
| Previous stroke/TIA | 199 (14.9) | 52 (16.4) | 147 (14.4) | 0.392 |
| Previous PCI | 529 (29) | 104 (25.1) | 425 (30.2) | 0.042 |
| Previous CABG | 268 (14.6) | 71 (17) | 197 (13.9) | 0.111 |
| Echocardiographic characteristics | ||||
| LVEF (%) | 51.2 ± 12.5 | 49.8 ± 13.4 | 51.6 ± 12.2 | 0.05 |
| Mean aortic gradient (mmHg) | 51.9 ± 14.8 | 53.8 ± 14.4 | 51.4 ± 14.9 | 0.001 |
| Moderate or severe MR | 734 (42.8) | 189 (49.1) | 545 (40.9) | 0.004 |
| Moderate or severe AR | 508 (30.2) | 140 (36.8) | 368 (28.2) | 0.001 |
| Procedural characteristics | ||||
| Access | 0.324 | |||
| Femoral | 1492 (81.4) | 346 (83.2) | 1146 (80.8) | |
| Subclavian | 239 (13) | 46 (11.1) | 193 (13.6) | |
| Aortic | 98 (5.3) | 24 (5.8) | 74 (5.2) | |
| Predilation | 1404 (81.3) | 282 (71.4) | 1122 (84.3) | <0.001 |
| Valve-in-valve | 80 (4.4) | 43 (10.3) | 37 (2.6) | <0.001 |
| Prosthesis size (mm) | <0.001 | |||
| 23 | 36 (2) | 9 (2.2) | 27 (1.9) | |
| 26 | 845 (46.1) | 143 (34.3) | 702 (49.5) | |
| 29 | 810 (44.2) | 211 (50.6) | 599 (42.3) | |
| 31 | 143 (7.8) | 54 (12.9) | 89 (6.3) | |
| Procedural time (min) | 109.4 ± 52.8 | 123.1 ± 55.1 | 105.5 ± 51.6 | <0.001 |
- Note: Values are expressed as n/N of patients (%) or mean ± standard deviation.
- Abbreviations: AR, aortic regurgitation; BMI, body mass index; BPD, balloon post-dilation; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MR, mitral regurgitation; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PVL, paravalvular leak; RBBB, right bundle branch block; STS, society of thoracic surgeons; SVD, structural valve deterioration; TIA, transitory ischemic attack.
3.2 Procedural and in-hospital outcomes
Transfemoral approach was widely used (81.4%) in both groups with no differences regarding alternative approaches (Table 1). Patients undergoing BPD as compared to those not undergoing BPD received less frequently balloon pre-dilation (71.4% vs. 84.3%, p < 0.001). BPD was more frequently performed when the 29 mm (50.6% vs. 42.3%) and the 31 mm (12.9% vs. 6.3%) prostheses were implanted (p < 0.001) and when a second CoreValve was required as a bailout procedure for acute implant failure (10.3% vs. 2.6%, p < 0.001). The balloon sizes used for BPD for each transcatheter heart valve size are listed in Table 2. Six patients (16.6%) who received the 23 mm prostheses underwent BPD with a bigger balloon than the recommended by instructions for use (>20 mm), 82 patients (9.7%) who received the 26 mm prostheses (balloon >23 mm), 112 patients (13.8%) who received the 29 mm prostheses (balloon >26 mm), and 3 patients (2.1%) who received the 31 mm prostheses underwent BPD with a bigger balloon than the recommended by instructions for use (>28 mm) (Table 2).
| CoreValve | ||||
|---|---|---|---|---|
| 23 mm | 26 mm | 29 mm | 31 mm | |
| Balloons (mm) | ||||
| 18 | 1/36 (2.8%) | 1/845 (0.1%) | 0 | 0 |
| 20 | 1/36 (2.8%) | 7/845 (0.8%) | 0 | 0 |
| 22 | 2/36 (5.6%) | 28/845 (2.8%) | 1/810 (0.1) | 0 |
| 23 | 4/36 (11.1%) | 19/845 (2.2%) | 1/810 (1.7%) | 1/143 (0.7%) |
| 24 | 0 | 8/845 (0.9%) | 2/810 (0.2%) | 1/143 (0.7%) |
| 25 | 0 | 71/845 (8.4%) | 62/810 (7.7%) | 6/143 (4.2%) |
| 26 | 0 | 3/845 (0.4%) | 15/810 (1.9%) | 1/143 (0.7%) |
| 27 | 0 | 0 | 1/810 (0.1%) | 0 |
| 28 | 0 | 0 | 104/810 (12.8%) | 38/143 (26.6%) |
| 29 | 0 | 0 | 3/810 (0.4%) | 0 |
| 30 | 0 | 0 | 4/810 (0.5%) | 3/143 (2.1%) |
- Abbreviation: BPD, balloon post-dilation.
No significant differences between groups were observed neither in the incidence of any procedural complication nor in-hospital clinical outcomes (Supporting Information: Table 1).
Postprocedural echocardiography showed that patients undergoing BPD had higher incidence of at least moderate PVL as well as a higher incidence of more than mild mitral regurgitation (Supporting Information: Table 1).
3.3 Long-term clinical outcomes
At 6-year follow-up, no differences among patients undergoing BPD and those not undergoing BPD were found for any clinical outcome (Table 3 and Figure 1).
| Outcome | Overall (N = 1835) | BPD (N = 417) | No-BPD (N = 1418) | Hazard ratio (95% CI) | p Value |
|---|---|---|---|---|---|
| All-cause mortality | 937 (51.1) | 204 (48.9) | 733 (51.7) | 1.05 (0.9−1.22) | 0.557 |
| Cardiovascular death | 263 (14.3) | 67 (16.1) | 196 (13.8) | 1.25 (0.94−1.64) | 0.120 |
| MI | 49 (2.7) | 11 (2.6) | 38 (2.7) | 1.06 (0.54−2.07) | 0.874 |
| Stroke/TIA | 115 (6.3) | 18 (4.3) | 97 (6.8) | 0.67 (0.40−1.11) | 0.117 |
| Aortic surgery | 11 (0.6) | 3 (0.7) | 8 (0.6) | 1.32 (0.35−4.96) | 0.686 |
| New PM implantation | 568 (31.3) | 131 (31.7) | 437 (31.1) | 1.08 (0.89−1.31) | 0.442 |
| Valve thrombosis | 4 (0.2) | 2 (0.5) | 2 (0.1) | NA | NA |
| Endocarditis | 8 (0.4) | 2 (0.5) | 6 (0.4) | NA | NA |
- Abbreviations: BPD, balloon post-dilation; CI, confidence interval; MI, myocardial infarction; PM, pacemaker; TIA, transitory ischemic attack.
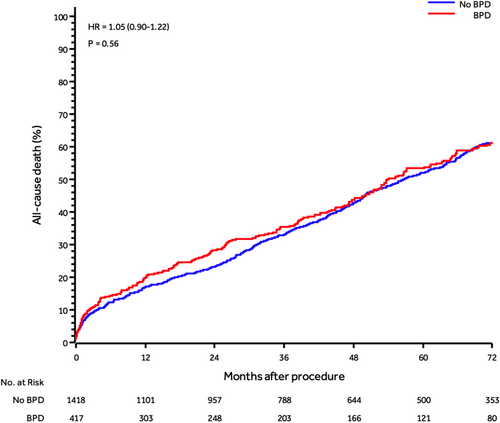
At multivariate analysis, age at procedure (HR: 1.02; 95% CI: 1.0−1.04, p = 0.019), creatinine >2 (mg/dL) (HR: 1.88; 95% CI: 1.46−2.42, p < 0.001), diabetes (HR: 1.36; 95% CI: 1.12−1.64, p = 0.002), prior stroke/TIA (HR: 1.36; 95% CI: 1.07−1.71, p = 0.011), severe chronic obstructive pulmonary disease (HR: 1.43; 95% CI: 1.17−1.74, p < 0.001), and left ventricular ejection fraction <50% (HR: 1.27; 95% CI: 1.06−1.52, p = 0.011), were associated with an increased risk of 6-year all-cause death. Instead, body mass index was associated with a decreased risk of 6-year all-cause death (HR: 0.97; 95% CI: 0.95−0.99, p = 0.014) (Supporting Information: Table 2).
3.4 Long-term SVD and echocardiographic outcomes
At 6-year follow-up echocardiographic data was available in 174 patients (40.2%). Six patients undergoing BPD and 30 patients not undergoing BPD experienced SVD within 6 years from implant with no statically significant differences between groups (1.4% vs. 2.1%, p = 0.381) (Table 4). Assuming death as a competing risk that can prevent SVD to happen, actual analysis resulted in a 6-year CIF for SVD of 1.04% (0.29%−2.84%) in patients undergoing BPD and 0.73% (0.34%−1.38%) in patients not undergoing BPD (Supporting Information: Figure 1).
| Overall (N = 1835) | BPD (N = 417) | No-BPD (N = 1418) | p Value | |
|---|---|---|---|---|
| SVD | 36 (2) | 6 (1.4) | 30 (2.1) | 0.381 |
| Intravalvular leak | 0.906 | |||
| 0 | 109/127 (85.8) | 31/34 (91.2) | 78/93 (83.9) | |
| 1 | 15/127 (11.8) | 3/34 (8.8) | 12/93 (12.9) | |
| 2 | 2/127 (1.6) | 0/34 (0) | 2/93 (2.2) | |
| 3 | 1/127 (0.8) | 0/34 (0) | 1/93 (1.1) | |
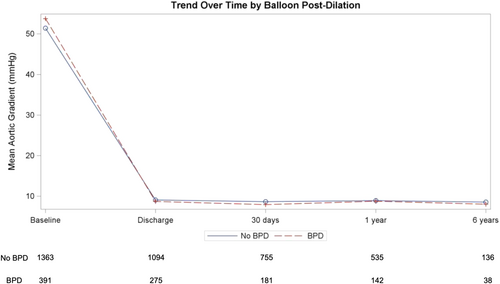
| Mean aortic gradient (mmHg) | 8.4 ± 5.7 | 8.4 ± 5.7 | 8.4 ± 5.7 | 0.441 |
| Moderate or severe MR | 62/164 (37.8) | 16/36(44.4) | 46/128 (35.9) | 0.352 |
| Moderate or severe PVL | 18/169 (10.7) | 4/39 (10.3) | 14/130 (10.8) | 1 |
| LVEF (%) | 54.9 ± 8.4 | 53.8 ± 8.9 | 55.2 ± 8.2 | 0.683 |
- Note: Values are expressed as n/N of patients (%) or mean ± standard deviation.
- Abbreviations: BPD, balloon post-dilation; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; PVL, paravalvular leak; SVD, structural valve deterioration.
On echocardiography, no differences between groups were found in terms of mean aortic gradient, intravalvular, and PVL at 6 years follow-up (Table 4). Changes in mean aortic gradient by group over time are depicted in Figure 2.
4 DISCUSSION
- 1)
No differences between patients undergoing BPD and those not undergoing BPD after TAVI were found in terms of all-cause mortality, cardiovascular death, stroke/TIA, valve thrombosis, and endocarditis at 6-year follow-up.
- 2)
After adjusting for confounders, BPD was not associated with higher rates of 6-year all-cause mortality.
- 3)
BPD after TAVI was not associated with an increased risk of SVD at long-term follow-up.
PVL following TAVR results from incomplete prosthesis apposition to the native annulus due to the extent of calcification or annular eccentricity, undersizing of the device, and/or malpositioning of the valve.14-16 Numerous studies have shown significant rates of moderate-to-severe PVL after TAVI, which has been associated with an increased 1-year mortality.5, 17, 18 However, the impact of mild PVL on mortality remains controversial. Some studies have suggested that mild PVL after TAVI leads to benign clinical outcomes,19-21 while other investigations have demonstrated that mild PVL also results in significantly higher long-term mortality.22, 23 One potential explanation for this difference is variability in the assessment of PVL severity, resulting in different patient populations across studies. 24
BPD is the first-line strategy to reduce the degree of PVL after TAVI. BPD has been shown to obtain optimal prosthesis frame expansion, reduce the severity of PVL, improve effective orifice area, and reduce patient-prosthesis mismatch.25, 26 However, BPD is not exempt from risks. Aortic annulus rupture, cerebrovascular events, conduction disorders, valve embolization, and prosthesis damage have been proposed as potential complications following BPD after TAVI. Although early studies had suggested a higher risk of cerebrovascular events after BPD, more recent analysis have shown that BPD was safe and not associated with increased risk of stroke, aortic root injury and mortality.11, 27 However, follow-up duration of the aforementioned studies is limited (12 months) and no prior study had evaluated the impact of BPD on long-term clinical outcomes and valve durability. The present investigation provides the first evidence about long-term safety of BPD. In our study, BPD was not associated with an increased risk of all-cause mortality, cardiovascular death, cerebrovascular events, myocardial infarction, and new pacemaker implantation at 6-year follow-up.
Another major concern after BPD is long-term SVD. SVD is an acquired intrinsic prosthesis valve abnormality due to deterioration of the leaflets or supporting structures resulting in valve hemodynamic dysfunction, manifested as stenosis or regurgitation.28, 29 Tissue disruption over time because of mechanical stress in conjunction with abnormal flow shear stress at the surface of valve leaflets, collagen fiber disruption, and tissue calcification have been suggested as mechanisms of SVD.28, 29 Many conditions may impact on the natural history of SVD such as remaining valve calcification, incomplete valve expansion and BPD.28 BPD has been postulated to damage the pericardial leaflets originating leaflet tears or flails and leading to local prothrombotic conditions such as exposure of tissue factor (secondary to dilation-related endothelial disruption).30 These local conditions may lead to thrombosis around the prosthesis and potentially cause valve thrombosis.30 However, as shown in our investigation, no statistically significant differences were found at 6-year follow-up between patients undergoing BPD and those not undergoing BPD in terms of valve thrombosis. Of note, valve thrombosis diagnosis was based on clinical suspicion and no systematic evaluation with computed tomography was performed to detect subclinical leaflet thrombosis. Notwithstanding, the impact of subclinical leaflet thrombosis on subsequent thromboembolic complications and valve function has reported conflicting results.31 Similarly, no statistically significant differences were observed between groups in other adverse events which could have been potentially originated at long-term follow-up after BPD such as endocarditis or intravalvular leak. In addition, changes in mean aortic gradient over time were similar between groups. All these findings are concordant with the lack of statistically significant differences in SVD among patients undergoing BPD and those not undergoing BPD at 6-year follow-up. These findings remained consistent when assuming death as a competing risk that can prevent SVD to happen (Figure 3).
BPD is a key procedural aspect in the therapeutic armamentarium of patients undergoing valve-in-valve. In this setting, BPD at very high pressures to modify or rupture the structure of a previously implanted surgical valve is performed. This strategy may expose the recently implanted valve leaflets to the high pressures of the ballooning, which could lead to tears and potentially impair durability. Despite our investigation was not specifically performed in patients undergoing valve-in-valve, 10.3% of the patients in the BPD group underwent more than one valve implantation as a bailout procedure for acute implant failure. Though our results might shed light in this setting, the impact of BPD on long-term valve durability in patients undergoing bioprosthetic valve fracture yet needs to be determined.
In summary, the findings of the present investigation are of utmost clinical relevance, supporting the role and safety of BPD after TAVI at long-term follow-up.
5 LIMITATIONS
The present study should be interpreted considering some limitations. First, the indications and technique of BPD, as well as balloon choices, were left to the discretion of the physician of each center. Therefore, detailed reasons to perform BPD were not available. Second, clinical events were reported under the responsibility of all the participating centers without a validation from a central independent committee and no CoreLab has been involved in the evaluation of the echocardiographic data neither for the pre-BPD procedure nor for the post-BPD evaluation. Third, it cannot be excluded that the lack of statistically significant differences between groups for some endpoints (i.e., new pacemaker implantation) might be due to small sample size. Fourth, the higher than expected number of patients lost at follow-up might have contributed to low event rates (i.e., endocarditis, valve thrombosis). Fifth, the number of patients surviving and with an echocardiographic follow-up available at 6 years was relatively small but, to the best of our knowledge, this study represents the only one aimed to evaluate the long-term impact of BPD reported so far. Sixth, long-term SVD follow-up was performed in a cohort of surviving patients, with death possibly exerting a competing risk that may have biased our results. To limit this effect, we reported both actuarial and actual estimates, as recommended by the EAPCI/ESC/EACTS consensus statement. Seventh, whether these results can be extrapolated to lower risk patients and different prosthesis yet needs to be determined.
6 CONCLUSIONS
BPD following the implantation of the self-expanding CoreValve and Evolut R prosthesis does not seem to be associated with an increased risk of adverse clinical outcomes or SVD at 6-year follow-up.
ACKNOWLEDGMENTS
Open access funding provided by BIBLIOSAN.
CONFLICTS OF INTEREST STATEMENT
Dr. Jorge Sanz has received speaking fees from Cordis, Terumo, Boston, and Medtronic. Dr. Marco Barbanti is consultant for Medtronic, Boston Scientific, and Edwards LifeSciences. Dr. Matteo Montorfano has recieved consultant fees from Abbott, Boston Scientific, Edwards, Kardia, and Medtronic. Dr. Corrado Tamburino is consultant for Medtronic. Dr. Bernhard Reimers has received speaking honoraria from Boston Scientific. The remaining authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.