Mitral valve-in-valve with 4D intracardiac echocardiography: Procedural and imaging technique
Abstract
Transcatheter mitral valve-in-valve (ViV) has emerged as a safe and effective therapeutic option for patients with a degenerated mitral bioprosthesis. As procedural techniques mature and operator experience improve, there is a push to adopt a “minimalist” approach of using conscious sedation instead of general anesthesia for faster recovery. The heavy reliance on fluoroscopy for ViV deployment makes feasible the use of intracardiac echocardiography (ICE) instead of transesophageal echocardiography for other procedural imaging requirements. We hereby use a case example to illustrate a step-by-step approach of using four-dimensional ICE to guide transcatheter mitral ViV under conscious sedation.
1 INTRODUCTION
Transcatheter mitral valve-in-valve (ViV) is a safe and effective alternative to open-heart surgery for patients with failed surgical bioprostheses.1, 2 The procedure has thus far been performed predominantly with fluoroscopic and transesophageal echocardiographic (TEE) guidance. Intracardiac echocardiography (ICE) has emerged as an alternate imaging modality to TEE for structural interventions with the benefit of avoiding general anesthesia (GA) as well as endotracheal and esophageal intubation. With the advancement of ICE technology, the prospect of supporting more complex interventions has become a possibility, which could not have been achieved in the past with two-dimensional (2D) ICE, as the imaging planes cannot be directly oriented to the mitral plane.3-5 We present a step-by-step approach of using four-dimensional (4D) ICE to provide imaging support for mitral ViV performed under conscious sedation with a case example as illustration.
2 CASE REPORT
The patient was an 80-year-old male who had a history of severe degenerative mitral regurgitation for which he underwent a surgical mitral valve repair in 2005 and a mitral valve replacement in 2010 with a 29 mm Carpentier-Edwards Perimount Theon (Edwards Lifesciences) bioprosthesis. His other significant co-morbidities included atrial fibrillation, complete heart block with a permanent pacemaker and a history of a difficult intubation that resulted in pharyngeal laceration requiring surgical repair. He presented to our institution with progressive dyspnea on exertion and transthoracic echocardiogram (TTE) showed severe bioprosthetic mitral stenosis (mean transmitral pressure gradient of 14 mmHg), impaired left ventricular ejection fraction of 40% and severe pulmonary hypertension (pulmonary artery systolic pressure of 63 mmHg). In view of his age, co-morbidities and two prior sternotomies, he was deemed to be at high-risk for another open-heart surgery and was hence evaluated for transcatheter mitral ViV replacement.
Preprocedural computed tomography showed adequate neo-left ventricular outflow tract (LVOT) clearance for implantation of a 29 mm SAPIEN 3 (Edwards Lifesciences) valve. Due to a history of difficult intubation and pharyngeal trauma, we decided to perform the procedure under conscious sedation with 4D ICE guidance. Vascular access was obtained in both femoral veins with an 11 French sheath in the left femoral vein for the NuVision (Biosense Webster Inc) 4D ICE catheter and an 8.5 French transseptal sheath in the right femoral vein. Transseptal access was guided by ICE and we obtained a posterior and inferior puncture for optimal trajectory of device crossing through the septum and mitral valve (Table 1A). After which a 0.035” Amplatz Super Stiff guidewire (Boston Scientific, Malborough, MA) was placed in the left upper pulmonary vein to facilitate atrial septostomy using a 12 × 20 mm balloon (Table 1B). Next the 4D ICE catheter was introduced into the left atrium (LA) through the created atrial septal defect (ASD) to interrogate the left atrial appendage and mitral valve (Table 1C). Real-time multiplanar three dimensional (3D) imaging of the trileaflet surgical bioprosthesis showed an immobile leaflet and another that was severely restricted (Table 1D). A 12 French Oscor Destino steerable guide sheath (Oscor Inc, Palm Harbor, FL) was brought into the LA and a Safari2 (Boston Scientific) preshaped guidewire was introduced into the left ventricle. ICE imaging was used to ensure that the wire crossed within the surgical stent frame (Table 1E). The steerable guide catheter was exchanged out for the 16 French Edwards eSheath and the 29 mm SAPIEN 3 valve was brought across the atrial septum into the LA. Positioning and deployment of the SAPIEN 3 valve was done fluoroscopically by aligning with the radioopaque stent frame (Table 1F). The patient's own permanent pacemaker was used to rapid pace during deployment. Post-deployment assessment showed a well-seated SAPIEN 3 valve with no mitral regurgitation and a final transmitral mean pressure gradient of 1 mmHg (Table 1G). The ICE catheter was brought back to the right atrium to interrogate the ASD (Table 1H). There was bidirectional shunting but in view of adequate oxygenation, the decision was made not to close the ASD in an effort to preserve future transseptal LA access. TTE showed no LVOT obstruction and no pericardial effusion. The right femoral venous access was closed with a figure-of-8 suture and the left via manual compression. He was monitored on the floor overnight and discharged the next day. At 1-month follow-up, he was asymptomatic and TTE showed a normal functioning mitral prosthesis with no stenosis or regurgitation and the ASD was no longer seen.
| Procedural Step | Fluoroscopy and Intracardiac Echocardiography | Comments |
|---|---|---|
| (A) Transseptal puncture | 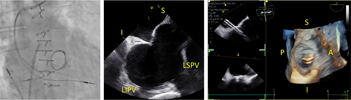 |
S—superior; I—inferior; A—anterior; P—posterior; LSPV—left superior pulmonary vein; LIPV— left inferior pulmonary vein |
| (B) Atrial septostomy | 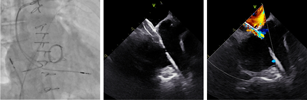 |
|
| (C) Left atrial appendage assessment | 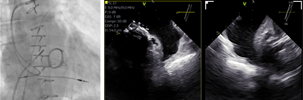 |
|
| (D) Mitral valve assessment | 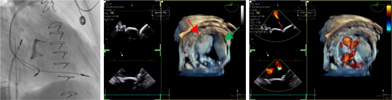 |
|
| (E) Wire crossing into left ventricle | 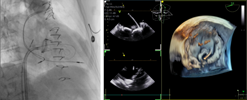 |
|
| (F) Valve positioning and deployment | 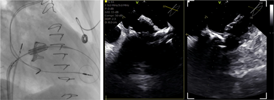 |
|
| (G) Post-deployment assessment | 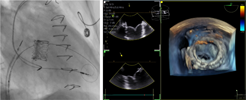 |
|
| (H) ASD assessment | 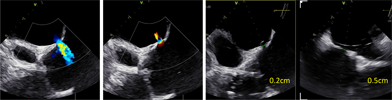 |
|
- Abbreviations: ICE, intracardiac echocardiography; 3D, three dimensional.
3 DISCUSSION
The positioning and deployment of transcatheter heart valves in mitral ViV procedures have relied predominantly on fluoroscopic landmarks of the surgical heart valves but there are other aspects of the procedure that would benefit from echocardiographic guidance. TEE provides excellent visualization of the interatrial septum, mitral valve and LA structures which makes it an ideal imaging modality for precise transseptal puncture and periprocedural guidance in mitral ViV. ICE has recently gained traction in the guidance of contemporary structural interventions. The ability of the ICE catheter to negotiate through vascular channels and cardiac chambers allows the imaging probe to be brought into close proximity of areas of interest for optimal visualization. The initial imaging limitations of 2D ICE have been overcome by the advent of 4D capabilities such as multiplanar reconstruction, electronic steering, and real-time 3D imaging. This allows for its guidance in more complex structural interventions, which could only be achieved in the past with TEE. Although generally considered a safe procedure, probe insertion and manipulation in the gastrointestinal tract during TEE is not without risks.6 Anatomical constraints that limit TEE imaging view (hiatal hernia) or hindrance to probe manipulation (esophageal stricture or varices) may also necessitate the use of ICE. With use of ICE and avoidance of TEE, the procedure can be performed with conscious sedation rather than GA. The use of conscious sedation for transcatheter aortic valve replacement has shown to be associated with improved outcomes and a faster recovery.7
4 CONCLUSION
We herein present a step-by-step approach of using 4D ICE for procedural imaging in transcatheter mitral ViV. In our opinion, the imaging quality of 4D ICE is comparable to TEE and adequate for the purpose of this procedure, in addition to eliminating the risks of TEE and GA. Using 4D ICE has the potential to be the standard of care for mitral ViV but further studies to demonstrate its safety and efficacy in larger numbers are required to back up this promise.
CONFLICT OF INTEREST STATEMENT
Dr. Lim reports receiving research grant support from Abbott Vascular, Boston Scientific, Corvia, Edwards Lifesciences, Medtronic, V Wave, and WL Gore as well as consultancy fees from Philips, Venus and Valgen.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.