Anoikis in cancer: The role of lipid signaling
Abstract
Malignantly transformed cells must alter their metabolic status to stay viable in a harsh microenvironment and maintain their ability to invade and spread. Anoikis, a specific detachment-related form of apoptotic cell death, is a potential barrier to cancer cell metastasis. Several molecular/pathway alterations have been implicated in preventing anoikis in metastatic cancers. Specific alterations in the lipid metabolism machinery (such as an increase in fatty acid uptake and synthesis) and modifications in the carbohydrate and amino acid metabolism are partially identified mechanisms associated with the anoikis resistance in various types of cancers, among other survival benefits. Following a summary of the molecular basis of the anoikis pathway, its resistance mechanisms, and the fundamentals of lipid metabolism in cancer, this article aims to elucidate the impact of lipid metabolism deviations recruited by cancer cells to escape anoikis.
1 INTRODUCTION
Despite significant and remarkable advancements in the detection and management of malignant neoplastic diseases, cancer remains a significant healthcare burden, with approximately 19.3 million newly diagnosed patients (among which female breast and lung cancer were the most prevalent) and 10 million cancer-related deaths worldwide in 2020 (Ferlay et al., 2021). In addition to a limited life expectancy, cancer patients always experience a number of somatic and psychological complications (Caruso et al., 2017; Corbett et al., 2020); even if they are survivors from a young age (Phillips et al., 2015). Among the specific characteristics of cancerous cells known as “hallmarks” of the disease, the ability of these cells to invade surrounding tissue and metastasize remotely is widely recognized as the leading cause of morbidity and mortality due to malignancy (Fares et al., 2020; Fouad & Aanei, 2017). Considering that the loss of a single cell's junction with adjacent cells and tissue matrix contributes to cell death (Paoli et al., 2013), a metastasizing malignant cell must maintain its viability and proliferative ability to overcome the metastatic cascade steps and seed a distant site (Fares et al., 2020). Detachment of a cell from its surrounding elements recruits proapoptotic pathways to activate caspases and apply apoptosis (via either an intrinsic or extrinsic pathway); this type of specific anchorage-induced apoptosis is known as “anoikis (Taddei et al., 2012). Unfortunately, some cancer cells use multiple advantages to overcome anoikis, such as obtaining mesenchymal characteristics and converting energy metabolism machinery, which is regarded as a formidable advantage (Kim et al., 2012; Paoli et al., 2013; Sakamoto & Kyprianou, 2010). Among these benefits, the metabolic reprogramming of lipid products has been studied as a method for preventing anoikis (Pacilli et al., 2013; Sawyer et al., 2020). Studies that uncover new aspects of cancer biology, particularly metabolic alterations in malignant tissues, have yielded promising therapeutic approaches (Feng et al., 2019; Vander Heiden & DeBerardinis, 2017). This paper aims to summarize the principles of anoikis mechanism and anoikis resistance in cancer development, review the advantage of lipid metabolism reprogramming in cancer cells, explain the effect of this reprogramming on anoikis avoidance by malignant cells, and discuss the future clinical applications of these issues in reducing the metastatic potential of cancer cells.
2 ANOIKIS AS A SPECIFIC FORM OF APOPTOSIS
Apoptosis, the programmed and highly controlled “falling down” and death of cells for the preservation of tissue homeostasis, has been identified for nearly half a century, and its role in normal human development and pathologies such as autoimmunity and cancer has been evidenced (DeLong, 1998; Kerr et al., 1972). In addition to apoptosis, autophagy and programmed necrosis have been implicated in the self-termination of cell lives (Bialik et al., 2018; Wu et al., 2012). Similar to other biological mechanisms, apoptosis consists of a series of sequential events. Although these pathways may exhibit cross-links, they possess unique properties (Elmore, 2007; Hongmei, 2012). Caspases are an essential mediator in both intrinsic and extrinsic apoptotic pathways among the molecular components of apoptotic pathways (Elmore, 2007; Hongmei, 2012; Mohana-Kumaran et al., 2014). However, it is now apparent that apoptosis can occur in a caspase-independent manner (Hongmei, 2012). The ultimate consequence of both pathways is packaging degraded intracellular components into “apoptotic bodies,” which are scavenged by tissue cells such as macrophages (D'Arcy, 2019).
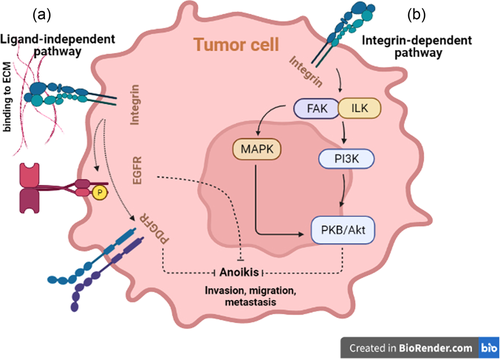
Conventionally, anoikis is attributed to the induction of apoptosis due to a single cell's detachment from neighboring cells and/or the surrounding matrix or engagement with an unfit microenvironment (Figure 1) (Grossmann et al., 2001; Paoli et al., 2013; Taddei et al., 2012). Previous research has shown that a cell's viability is maintained by recruiting pro-survival and suppressing apoptotic/anoikis pathways when adequately anchored to the surrounding extracellular matrix (ECM) or adjacent cells (Chiarugi & Giannoni, 2008; Taddei et al., 2012). In a normal microenvironment, cells are protected from apoptosis by integrin- and cadherin-related prosurvival signal transduction due to cell–ECM and cell–cell adhesion, respectively. Specific integrins, for instance, can recruit focal adhesion kinase (FAK), integrin-linked kinase, and their downstream phosphatidylinositol 3 phosphate kinase (PI3K) to activate protein kinase B (PKB/Akt), which is responsible for cell proliferation and repression of anoikis-inducing enzymes (Chiarugi & Giannoni, 2008; Hemmings & Restuccia, 2015; Taddei et al., 2012). In addition to the PI3K–PKB/Akt pathway, FAK can activate the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) pathway, which exerts a similar protective effect against anoikis; however, this pathway can be activated independently by the caveolin1-mediated effect of integrin (Guo et al., 2020; Paoli et al., 2013). Furthermore, the aforementioned survival pathways can cause anoikis-exerting mediators to be inactivated (Chiarugi & Giannoni, 2008). ECM-anchored integrin activates growth factor receptor downstream pathways (e.g., epithelial growth factor receptor [EGFR], platelet-derived growth factor receptor, and insulin receptor) independently of their activating ligands, resulting in anoikis suppression (Figure 2) (Chiarugi & Giannoni, 2008; Paoli et al., 2013). Despite integrins' significant role in anoikis suppression, it is shown that the mentioned prosurvival pathways can be preserved in ECM-detached cells that are still anchored to each other (Hofmann et al., 2007). Both intrinsic and extrinsic apoptotic pathways can be used to establish anoikis (Chiarugi & Giannoni, 2008). The Bcl-2 family of molecules is unavoidably involved in activating and inhibiting anoikis (K. Tan et al., 2013).
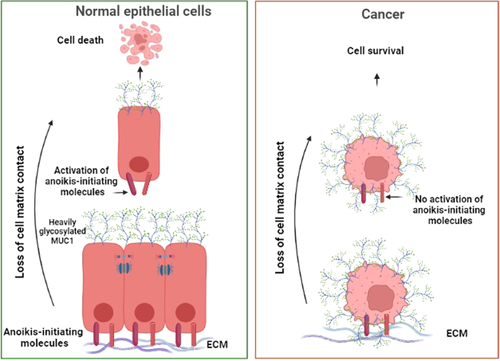
Following the separation of a single cell from the ECM, Bid and Bim (two BH3-only proapoptotic Bcl-2 family members) are activated, causing Bax and Bak (two multidomain proapoptotic Bcl-2 family members) to form oligomers within the outer membrane of the mitochondria, resulting in membrane perforation and the entry of cytochrome c, as well as other agents, into the cytoplasm. Subsequently, the apoptosome is formed by the assembly of cytochrome c, the protease activating cofactor Apaf, and caspase-9, which activates caspase-3, degrades the protein targets, and indirectly causes DNA fragmentation and intrinsic anoikis (Chiarugi & Giannoni, 2008; Grossmann et al., 2001; Paoli et al., 2013). Despite proapoptotic members of the Bcl-2 family, antiapoptotic members of this family, such as Mcl-1, Bcl-2, and Bcl-xL, aim to suppress anoikis. Mcl-1 activity, for example, neutralizes the Bim or Bak in the outer membrane of mitochondria (Chunhacha et al., 2012; Milot & Filep, 2011). As another example, Bcl-2 prevents Bak and Bax oligomerization after binding to them, inhibiting them and preserving mitochondrial membrane integrity (Chiarugi & Giannoni, 2008). Thus, by inhibiting the antiapoptotic Bcl-2 family, the mitochondrial-dependent intrinsic pathway can be strengthened even further.
Cells can also establish anoikis using the extrinsic apoptotic pathway. During this phenomenon, the membrane death receptor Fas and its ligand are upregulated due to cell detachment, resulting in Fas-associated death domain activation and downregulation of inhibitory molecules, which then activates caspase-8 via the function of the death-inducing signaling complex (DISC). Afterward, caspase-8 activates caspase-3 and induces anoikis in a manner similar to an intrinsic pathway. Caspase-3, on the other hand, may oligomerize Bad and Bak by truncating Bid, resulting in the interaction of extrinsic and intrinsic pathways (Chiarugi & Giannoni, 2008; Taddei et al., 2012). Intriguingly, the extrinsic pathway can be recruited by the proximity and subsequent activation of Fas receptors on the membrane caused by the rounding of the detached cells' shape (Paoli et al., 2013). The extrinsic pathway, like the intrinsic pathway, can be regulated by inhibitory molecules. Namely, FLICE-inhibitory protein (FLIP), which has a similar structure to caspase-8, has a higher affinity for DISC and competes with caspase-8, preventing its activation (Simpson et al., 2008).
Notably, anoikis can occur independently of the activity of caspases. Loss of integrin attachment releases the mitochondrial mediator Bit-1 into the cytoplasm, causing anoikis in which caspases play no role. This pathway does not appear to be inhibited by the antiapoptotic factors mentioned previously (Jan et al., 2004; X. Yao et al., 2014).
The formation of organs during embryonic development and the healing of wounds are two examples of physiological processes in which normal cells must migrate away from their current location (Guadamillas et al., 2011). The ability of these cells to acquire mesenchymal characteristics enables them to avoid anoikis and successfully migrate to a new location. In the case of malignancies, however, cancerous cells take advantage of this benefit to maintain their viability after losing connection with the primary site, circulating in the lymphatics or blood, and seeding in a distant foreign microenvironment. The fatal consequence is tumor metastasis (Guadamillas et al., 2011; Simpson et al., 2008).
3 ANOIKIS RESISTANCE AND CANCER DEVELOPMENT
The ability to metastasize is believed to be the feature that makes transformed cells “malignant” and contributes to cancer-related morbidity and mortality (Fidler, 1991). As mentioned previously, the ability of cancer cells to survive and maintain their proliferative potential after detachment from the primary site is essential for establishing a successful metastatic process (Nagaprashantha et al., 2011; Simpson et al., 2008). Therefore, multiple types of invasive metastatic cancers employ diverse mechanisms to acquire and maintain anoikis resistance. The following are examples of mechanisms utilized by various cancers to evade anoikis.
A family of caspase inhibitor of apoptosis proteins (IAPs), including X chromosome-linked inhibitor of apoptosis (XIAP), has been identified. It has been reported that in metastatic prostate cancer, XIAP inhibits the effector caspases, caspase-3, and caspase-7, as well as caspase-9, resulting in anoikis resistance; therefore, XIAP inhibition has led to prostate cancer cells' increased sensitivity to anoikis (Berezovskaya et al., 2005; Simpson et al., 2008). Additionally, Zhou, et al. (2017) demonstrated that XIAP is stabilized by USP-11 via binding and deubiquitylation, resulting in resistance to anoikis and tumor progression in breast cancer. P. Zhang et al. (2016) demonstrated that by using tetraiodothyroacetic acid, which antagonizes l-thyroxin (T4), the metastatic potential of prostatic cancer cell lines is reduced due to the downregulation of XIAP as well as the inhibition of the matrix metalloproteinase-2 and MAKP/ERK signaling pathways. Additionally, it is evident that XIAP and survivin (another member of IAPs) are activated by the nuclear factor-κβ (NF-κβ), resulting in anoikis resistance. However, the role of NF-κβ in promoting anoikis resistance is not confined to this case (Frisch et al., 2013; Mehrotra et al., 2010).
NF-kβ is a transcription factor that regulates the expression of multiple genes involved in inflammation pathways, tumor proliferation and survival, angiogenesis, and metastasis and is regarded as a crucial factor in cancer cells' utilization of inflammatory cytokines to suppress the host immune system (Aggarwal & Sung, 2011). Particular signals activate IKK before phosphorylating and detaching NF-kβ's regulatory protein, IkB (Tajbakhsh et al., 2019). The PI3K/Akt pathway is activated due to NF-kβ function (Paoli et al., 2013). This prosurvival pathway is responsible for tumor growth and metastasis and is a therapeutic target in cancer treatment (Jiang et al., 2020). This pathway suppresses the anoikis by inhibiting Bim, Bad, and procaspase-9 through their phosphorylation (Paoli et al., 2013; Taddei et al., 2012). In addition, other antiapoptotic molecules that are upregulated by NF-kβ and play a role in anoikis resistance are Bcl-2, Bcl-xL, FLIP, and neurotrophic receptor tyrosine kinase receptor B (TrkB) (Tajbakhsh et al., 2019). In light of FLIP's inhibitory effect on extrinsic anoikis, it is essential to note that this molecule is overexpressed in cancer cells, and its inhibition has been proposed as a potential therapeutic strategy (Kim et al., 2012; Simpson et al., 2008). TrkB has been shown to play a unique role in the development and metastasis of cancers such as colorectal, cervical, gastric, and non-small cell lung cancer (Okamura et al., 2012; Yu et al., 2010; Yuan et al., 2018). Yuan et al. (2018) demonstrated that TrkB and its downstream pathways are generally upregulated in cervical cancer compared to the normal cervical epithelium and that these cells exhibited increased anoikis resistance. The study by Dawson et al. (2015) on colorectal cancer (CRC) samples also revealed that high cytoplasmic/membranous TrkB expression was positively correlated with tumor size and grade and negatively correlated with caspase-3 levels. In “budding” cells of CRC tissue, cytoplasmic/membranous TrkB expression was elevated, indicating anoikis resistance and epithelial-to-mesenchymal transition (EMT) phenotype.
Acquiring mesenchymal characteristics is essential for the successful migration and nesting of pluripotent cells during embryogenesis and development so that these cells can utilize particular advantages to ambulate and avoid anoikis (Heerboth et al., 2015; Nakaya & Sheng, 2013). In addition, EMT helps cancer cells overcome anoikis during metastatic cascades (Heerboth et al., 2015). For example, following EMT induction, cells lose the anchorage protein E-cadherin and simultaneously upregulate N-cadherin, leading to β-catenin and PI3K/AKT pathway activation, respectively, resulting in the transcription of proliferation- and metastasis-related genes and anoikis resistance (Heerboth et al., 2015; Paoli et al., 2013; M. Singh et al., 2018). Moreover, the NF-κβ pathway may be activated during EMT and exert antianoikis effects (Frisch et al., 2013). NF-κβ and hypoxia-inducible factors (HIFs) can activate the Snail transcription factor during EMT, which, in turn, activates Akt and Bcl-xL and represses E-cadherin and Bid, resulting in anoikis resistance (Heerboth et al., 2015; Paoli et al., 2013). It is also known that activation of HIFs is a characteristic of EMT in cancer cells, leading to suppression of anoikis via EGFR upregulation and degradation of proapoptotic Bim and Bmf (Whelan et al., 2010). As stated previously, integrins are crucial to the viability of anchored cells; however, cancer cells can overcome anoikis by altering their integrin status. For instance, anoikis resistance can be achieved through the downregulation of α5 integrin mediated by HIF-1 (Unwith et al., 2015).
In addition to the mechanisms mentioned above, it is well known that microRNAs (miRNAs) may affect anoikis. Depending on the miRNA type, this effect can be either positive or negative. Mak et al. (2017) demonstrated that miR-141 downregulates the KLF12 gene, resulting in the upregulation of survivin in ovarian cancer. The upregulation of survivin also results in tumor metastasis and resistance to anoikis. X. Zhang et al. (2015) provided evidence that in hepatocellular carcinoma, the anoikis process can be accelerated by the activity of miR-26a, which targets the α5 integrin gene, ITGA5.
The TME differs from normal tissues; hence, cancer cells make various metabolic pathway changes to guarantee survival (Faubert et al., 2020). It is no surprise that cancer cells benefit from “metabolic remodeling” to resist anoikis. For example, alterations in glucose metabolism are well understood in tumor cells, which use the relatively inefficient anaerobic glycolysis mechanism to produce ATP even when oxygen is present (Yoshida, 2015). However, it was recently found that after matrix detachment, cancer cells prefer to consume glutamine rather than glucose by activating the AMP-activated protein kinase (AMPK)–nuclear factor erythroid 2-related factor 2 signal to increase glutamine uptake and sustain oxidative homeostasis, indicating that glutamine metabolism may be more advantageous for avoiding anoikis (Endo et al., 2020).
4 LIPID METABOLISM REPROGRAMMING IN CANCER CELLS
Oncologists have been interested in the study of cancer metabolism for the past half-century (Hanahan & Weinberg, 2011). In particular, reprogramming of lipids metabolism has been considered over the past decade (Y. Chen & Li, 2016). Cancer cells alter their metabolic pathways compared to healthy cells and undergo metabolic reprogramming to meet their abnormal metabolic requirements for proliferation and survival. Metabolic reprogramming is recognized as one of the defining characteristics of cancer cells (Hanahan & Weinberg, 2011; Pavlova & Thompson, 2016; Xing et al., 2015; F. Zhang, & Du, 2012). This abnormal metabolism is mediated by the most frequently activated oncogenic pathways in human cancers, such as K-ras or myc (Carracedo et al., 2013; Faubert et al., 2020; Gaglio et al., 2011), changes in cell signaling, primarily the PI3K–AKT (Carracedo et al., 2013; M. G. Vander Heiden et al., 2009) and limitations imposed by the tumor microenvironment (TME) (Boroughs & DeBerardinis, 2015). These metabolic alterations in cancer cells are responsible for cancer cells' growth, maintenance, proliferation, and tumorigenic properties (Hanahan & Weinberg, 2011; Labbé et al., 2019; Lunt & Vander Heiden, 2011).
Changing glucose metabolism and the Warburg effect led to the discovery of reprogramming of cellular metabolism in cancer cells. Otto Warburg observed in 1920 that most cancer cells rely on aerobic glycolysis. This metabolic change promotes the growth and proliferation of cancerous cells despite the presence of sufficient oxygen (DeBerardinis et al., 2008; Lunt & Vander Heiden, 2011). Even though carbohydrates and aerobic glycolysis are inefficient in ATP production per glucose molecule (Ma et al., 2014), this condition is predominantly utilized for anabolic processes (Gatenby & Gillies, 2004; Pelicano et al., 2006).
Previous studies of cancer bioenergetics have focused on abnormal glucose and glutamine metabolism (Boroughs & DeBerardinis, 2015; Gaglio et al., 2011). While metabolic reprogramming of lipid metabolism in cancer cells was overlooked in early cancer research (J. Huang et al., 2016), it is now widely accepted that lipid metabolism plays a crucial role in the pathogenesis of human cancers and cancer-related cachexia (Giudetti et al., 2019; J. Huang et al., 2016; Menendez & Lupu, 2007; Snaebjornsson et al., 2020; C.-H. Yao et al., 2016).
In addition to changes in glucose metabolism, the alteration of metabolic lipids (free fatty acids [FFAs], cholesterol, and phospholipids) is a crucial aspect of metabolic reprogramming in cancer cells (Y. Chen & Li, 2016; Jafari et al., 2019; Swinnen et al., 2006). Fatty acid synthase (FASN) expression is low in normal tissues and cells except for liver and adipose tissue (Menendez & Lupu, 2007). The expression of lipogenesis key enzymes such as acetyl-CoA carboxylase (ACC), FASN, stearoyl-CoA desaturase 1 (SCD1), and cholesterol synthesis pathways is elevated in cancer cells (Jafari et al., 2019; Swinnen et al., 2006). In ovarian (Y. Cai et al., 2015), colorectal (Zaytseva et al., 2012), breast, and prostate cancers (Currie et al., 2013), FASN enzyme expression or activity was also elevated. Consequently, increased lipogenesis is a characteristic of cancer cells. Thus, cancer cells typically exhibit a lipid-rich phenotype, with a high affinity for lipids and cholesterol stored in lipid droplets (LDs) (F. Zhang, & Du, 2012). As revealed by Raman spectroscopy imaging, high-resolution magic angle spinning, and Nile Red staining, higher LD amounts have also been observed in CRC (Bozza et al., 2010; Farese & Walther, 2009) and brain tumors (Opstad et al., 2008) compared to normal counterparts.
Continuous de novo lipogenesis is advantageous for cancer cells because it supplies membrane-building blocks, lipid signaling molecules, posttranslational modifications of proteins, and energy to support rapid cell proliferation (Jafari et al., 2019; C.-H. Yao et al., 2016).
While most normal human cells prefer exogenous lipid sources, it is interesting to note that cancer cells, like embryonic cells, prefer de novo synthesized FAs for their biological activities despite the abundance of extracellular fatty acids.
Although some cancer cells utilize exogenous FAs, Jafari et al. (2019) found that colon cancer cells provide the necessary conditions for growth and proliferation by increasing de novo FAs synthesis. However, Nieman et al. (2011) and C.-H. Yao et al. (2016) demonstrated an increase in exogenous fatty acid uptake by ovarian tumor cells and HeLa and H460 cells, respectively. Overall, it can be stated that different cancer cell types obtain lipids from various sources, depending on environmental conditions.
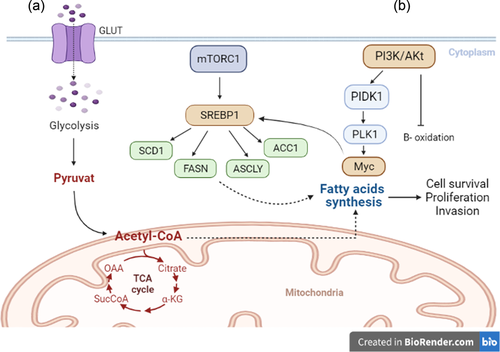
Oncogenes cause alterations in metabolic lipids in cancer cells. As Porstmann et al. (2005) showed that the oncogene Akt can activate sterol regulatory element-binding protein 1 (SREBP1), resulting in the induction of de novo synthesis of sterols and fatty acids (Yecies et al., 2011).
According to the study by Ricoult et al. (2016), PI3K and K-Ras induce lipogenesis by activating the target of rapamycin complex 1 (mTORC1), resulting in the overexpression of the essential transcription factor SREBP1 in cancer cells (Y. Sun et al., 2015). In addition to increased lipid synthesis in cancer cells, activating the mTORC1/SREBP pathway establishes a crucial link between oncogenic signaling and metabolic remodeling (Ricoult et al., 2016). mTORC1 facilitates lipogenesis by augmenting the expression of cell surface glucose transporters (Edinger & Thompson, 2002). By inhibiting B-oxidation and increasing FFAs synthesis, the PI3K/Akt pathway promotes increased lipogenesis during cell proliferation (DeBerardinis et al., 2006).
As a proto-oncogene, Myc is thought to encode the transcription factor SREBP1 and stimulate the tricarboxylic acid cycle, producing citrate as a precursor to fatty acid synthesis (FAS). Furthermore, Myc can activate FASN, ATP-citrate lyase, and SCD1, all of which are involved in converting citrate to fatty acids (Edmunds et al., 2014; Priolo et al., 2014).
Lipogenic enzyme inhibition has also been shown to reduce cell growth and tumor size in several types of cancer (Kuhajda, 2000). However, the relationship between fatty acid oxidation (FAO), glycolysis, glutaminolysis, and FAS in cancer cells has not been thoroughly studied. Recent research has demonstrated that FAO is essential for cancer cell survival and metastasis under metabolic stress conditions such as hypoxia, glucose deficiency, and energy-depleted environments (Kamphorst et al., 2013). FAO is an alternative pathway required in these cells for adaptation to energy source conservation, proliferation, and cancer cell survival (Buzzai et al., 2005; Deep & Schlaepfer, 2016; Pascual et al., 2017; Ricciardi et al., 2015; Shao et al., 2016) (Figure 3).
5 CANCER CELL ANOIKIS REGULATION BY TARGETING LIPID METABOLISM MEDIATORS
Lipid metabolism is involved in numerous cellular processes, including growth, proliferation, differentiation, survival, apoptosis, inflammation, motility, cell membrane homeostasis, response to chemotherapy, and drug resistance, through the production of various bioactive lipid molecules, such as signaling molecules (Hannun & Obeid, 2008; Krycer et al., 2010). Several studies have demonstrated the relationship between lipid metabolism and the anoikis process. Metabolic alterations and alterations closely related to apoptosis occur in cancer cells (Giordano et al., 2005). Several studies have demonstrated the relationship between lipid metabolism and the anoikis process. Metabolic alterations and alterations closely related to apoptosis occur in cancer cells (Giordano et al., 2005).
5.1 Carnitine palmitoyltransferase isoforms and anoikis
The metabolic characterization of malignant cells reveals a correlation between high respiration rate and apoptosis (Dier et al., 2014). In this regard, anoikis suppression is attainable through increased fatty acid uptake and oxidation (X. Li et al., 2018; Y. Tan et al., 2018). Y. Tan et al. (2018) reported that upregulation of phosphatidylinositol transfer protein cytoplasmic 1 promotes CD36 (a fatty acid transporter) and CPT1B in metastatic gastric cancer, resulting in increased fatty acid uptake and oxidation, as well as anoikis resistance in the omental metastatic specimens.
Additionally, AMPK-dependent activation of carnitine palmitoyltransferase 1C increases FAO to produce NADH, FADH2, and ATP, which are necessary for tumor growth, metabolic stress, and resistance to mTORC1 inhibitors, thereby protecting the cells from death (Zaugg et al., 2011). CPT1 overexpression has also been linked to tumor progression in breast cancer (Gatza et al., 2014; Linher-Melville et al., 2011; Pucci et al., 2016), gastric cancer (S. Li et al., 2014), prostate cancer (Liu, 2006), hepatoma (Rodríguez-Enríquez et al., 2015), myeloma (Tirado-Vélez et al., 2012), and high-grade glioblastoma (Wakamiya et al., 2014) according to previous research. According to Sawyer et al. (2020) and Pike et al. (2011) studies, CPT1A and FAO contribute to anoikis resistance. Cross-talk with the BH3 family of proapoptotic proteins explains the antiapoptotic function of CPT1 (Giordano et al., 2005). According to the study by Zaugg et al. (2011), CPT1A knockdown in cancer cells decreases ATP production and increases their susceptibility to metabolic stress and apoptosis. Moreover, CPT1A knockdown induces apoptosis by inhibiting BCL2 (Chowdhury et al., 2018).
5.2 Cell-to-cell adhesion-dependent lipid alteration, lipid rafts, and anoikis
Adhesion between cells and their interaction with the ECM is crucial for cell growth and survival (Schafer et al., 2009). Due to impaired glucose transport, tumor-detached cells known as loss of attachments face ATP deficiency and cellular stress (Carracedo et al., 2013; Pike et al., 2011; Sawyer et al., 2020; Schafer et al., 2009). ECM detachment induces FAO metabolic reprogramming in high-grade serous ovarian cancer via AMPK or p53 (Buzzai et al., 2005; Sawyer et al., 2020). Increased FAO is also essential for protecting cancer cells against environmental stress and anoikis (Carracedo et al., 2013; Deep & Schlaepfer, 2016).
Lipid rafts have been reported to mediate integrin signaling. Thus, lipid raft modulation is associated with adhesion-dependent cell survival. In suspended cells, cell detachment initiates lipid raft internalization, inhibits Rac1 as a mediator of membrane lipid raft internalization, and activates a downstream effector (del Pozo et al., 2004). This study demonstrates that integrins control the localization of lipid rafts to regulate anchorage-dependent cell growth. FAK downregulation, cell detachment, lipid raft internalization, and anoikis-like cell death have been observed in A431 human cervical cancer cells due to lipid rafts' depletion from cholesterol (Y. C. Li et al., 2006; Park et al., 2009). Disruption of lipid raft also induces Src-mediated phosphorylation of caveolin-1 tyrosine residues, which is essential for lipid raft internalization. In contrast, lipid raft disruption stress induces HIF-1α expression via EGF receptor activation, which delays anoikis. Consequently, HIF-1α inhibition also induces anoikis (S.-H. Lee et al., 2009).
Aloe-emodin, an anthraquinone derivative, lipid raft fraction sphingolipid, and cholesterol content reduction may also mediate lipid raft alteration. This agent inhibits tumor cell adhesion by destroying lipid raft-associated integrin signaling pathways, including FAK recruitment to β1 integrin (Q. Huang et al., 2006) and sensitizing gastric and lung cancer cells to apoptosis (J. Cai et al., 2008; H.-Z. Lee et al., 2010). Furthermore, intact lipid rafts facilitate Akt recruitment and activate this pathway by accumulating phosphatidylinositol-3,4,5-triphosphate in the membrane, which is necessary for PI3K/Akt signaling activity (Lasserre et al., 2008). Lipid rafts disruption by cholesterol depletion also causes lipid raft internalization and Akt inactivation even in response to EGF stimulation; therefore, cholesterol replenishment reverses these effects, indicating that plasma membrane lipid raft localization is essential for Akt activation (Y. C. Li et al., 2006; Park et al., 2009).
5.3 FASN and anoikis
FASN upregulation in cancer cells has also been associated with the PI3K/AKT signaling pathway (Bandyopadhyay et al., 2005; Porstmann et al., 2005). PI3K/Akt signaling activation recruits multiple signaling proteins, including AKT. Then, AKT plays an important role against apoptosis and exerts its antiapoptotic activity by phosphorylating and inactivating downstream targets such as caspase-9 and Bad (Díaz-Montero et al., 2006; Franke et al., 2003). AKT also induces the expression of prosurvival genes such as Bfl-1 and IAP1/2 via phosphorylation of NF-κβ, which has an antiapoptotic effect (Porstmann et al., 2005). This signaling pathway is only essential in cancer cells under stress conditions and protects cells from anoikis (Díaz-Montero et al., 2006; Zhao et al., 2019).
In addition, it has been reported that increased FASN activity by ERK1/2/Bcl-xL induces anoikis resistance, metastasis, and cancer recurrence (T. Sun et al., 2019). In contrast, FASN inhibition by C75 in epithelial ovarian carcinoma has led to AKT inactivation and anoikis augmentation (Uddin et al., 2011).
5.4 SCD1 and anoikis
SCD1 is essential in many human cancers such as lung, breast, prostate, and clear cell renal cell carcinoma. It has been reported that SCD1 increases lipogenesis and inhibits FAO in lung cancer. Consequently, this enzyme activates and deactivates Akt signals and the AMPK pathway, favoring increased cell proliferation, invasiveness, survival, and, ultimately, tumorigenic potential (Noto et al., 2013). Noto et al. (2013) demonstrated that the SCD1 inhibitor MF-438 increased anoikis in Pe O11 spheroids. Immunofluorescence staining with the M30 CytoDEATH Fluorescein monoclonal antibody, which detects caspase-cleaved cytokeratin 18, revealed a significantly higher proportion of M30 marker-positive apoptotic cells in MF-438-treated spheroids compared to the control group (Noto et al., 2013). In addition, the small-molecule inhibitors MF-438, CAY10566, and SC-26196 reduced the sphere-forming efficiency of lung and ovarian cancer stem cells via anoikis activation and cellular damage (Peláez et al., 2020).
5.5 Lipid metabolism mediators, EMT induction, and anoikis
The EMT is a type of cellular differentiation that occurs during embryonic development. Regulated EMT forms organs and tissues during development and maintains their function, growth, and regeneration (T. Chen et al., 2017). However, EMT dysregulation leads to diseases such as maldevelopment, fibrosis, and malignancy (Son et al., 2017).
Previous research has found that inhibiting fatty acid synthetic enzymes such as FASN, 3-hydroxy-3-methylglutaryl-CoA reductase, and ACC inhibits EMT induction in breast cancer cell lines (R. Singh et al., 2015). However, another study found that inactivated ACC causes the accumulation of high acetyl CoA levels after phosphorylation in invading breast cancer cells, which can then play a role in epigenetic gene regulation via SMAD2 acetylation in both in vitro and in vivo models (Garcia et al., 2017). In addition, a study on kidney proximal tubular cells (HK-2 cell line) reported an increase in lipid content, a decrease in β-oxidation, and an increase in EMT-related gene expression in these cells, which were alleviated by ACC2-specific silencing (Xu et al., 2014). This study suggests that the lipid composition of tumor cells may facilitate EMT induction. In addition, activated ACC mediates the high energy consumption of tumor cells and aids in the EMT process while regulating the acetylation process of other molecular events. Overall, the EMT process and its important plasticity resulting from cellular transdifferentiations due to mesenchymal–epithelial transition are associated with tumor progression and metastasis (Brabletz et al., 2021; Pastushenko & Blanpain, 2019). Therefore, the mutual correlation between lipid metabolism, EMT induction, and resistance to anoikis could be viewed as a novel therapeutic strategy for improved cancer management and treatment.
6 CONCLUSION
Collectively, following detachment from their primary location, malignant cells tend to acquire and utilize anoikis resistance to establish a well-organized metastatic cascade. Alterations in lipid metabolism pathways, in conjunction with other mechanisms, are noteworthy emerging examples to consider. High levels of FAS, uptake, and β-oxidation have been observed in tumor cells exhibiting an anoikis-resistant phenotype. Given these findings and the additional role of lipid metabolism deviation in cancer development, using potential agents to suppress this phenomenon, such as small molecules or small interfering RNAs, appears beneficial for preventing regional and distant spread. Indeed, additional preclinical and bioinformatics research is required to identify the appropriate molecules/pathways for regulation.
AUTHOR CONTRIBUTIONS
Mortaza Raeisi: Methodology and writing – original draft. Mojtaba Zehtabi: Software. Kobra Velaei: Software and visualization. Parisa Fayyazpour: Writing – original draft. Negar Aghaei: Investigation and writing – original draft. Amir Mehdizadeh: Conceptualization and writing – review and editing.
ACKNOWLEDGMENT
The authors would like to thank the Hematology and Oncology Research Center for their invaluable assistance.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




