Interaction of KCNA5, CX43, and CX40 proteins in the atrial muscle of patients with atrial fibrillation
Abstract
The objective of the study was to investigate the expression levels of potassium voltage-gated channel subfamily A member 5 (KCNA5), connexin 43 (Cx43), and connexin 40 (Cx40) in the left atrial appendage of patients with atrial fibrillation (AF) and the interactions between them. We gathered tissue samples from patients with persistent AF and sinus rhythm and used fluorescence quantitative polymerase chain reaction to evaluate messenger RNA (mRNA) changes of KCNA5, Cx43, and Cx40. Then, we studied the protein levels of KCNA5, Cx43, and Cx40 by immunofluorescence and western blot analysis and the interactions between these proteins were identified by immunoprecipitation and immunofluorescence colocation, respectively. Compared with the control group, the mRNA and protein levels of KCNA5, Cx43, and Cx40 in the AF group were decreased and the positive expression of KCNA5, Cx43, and Cx40 protein was also decreased by immunofluorescence staining in the AF group. In addition, immunoprecipitation and immunofluorescence colocation revealed that KCNA5 was coexpressed with Cx43 and Cx40 proteins. The expressions of KCNA5, Cx43, and Cx40 were substantially downregulated in the myocardium of patients with AF and KCNA5 interacted with Cx43 and Cx40 proteins, respectively.
1 INTRODUCTION
Atrial fibrillation (AF) is a major risk factor for ischemic stroke and persistent AF can induce malignant arrhythmia and endanger life. In recent years, the incidence and prevalence of AF have been increasing year by year, posing a serious threat to human health (Lippi et al., 2021). Atrial remodeling is an important pathophysiological basis for the onset of AF (Nattel & Harada, 2014). Studies have reported that changes in ion channel membrane protein and gap junction protein expression play a crucial role in atrial remodeling.
Potassium voltage-gated channel subfamily A member 5 (KCNA5), which encodes the a-subunit of the ultra-rapidly activating delayed rectifier potassium channel (Kv1.5) predominantly expressed in human atria (Y. Yang et al., 2009). The Kv1.5 is considered to be essential to the expression of ultrarapid postponed rectifier (IKur) in the human heart and IKur is believed to be vital in the repolarization of human atrial myocytes (Feng et al., 1997; Olson et al., 2006; Y. Yang et al., 2009). Therefore, we speculated that KCNA5 might be involved in the pathological process of AF. At present, the pathogenic link between KCNA5 mutation and susceptibility to AF was confirmed both in vitro and in vivo experiments (Olson et al., 2006), implicating KCNA5 as an ideal target for the treatment of AF (Blass et al., 2009). However, the specific regulatory mechanism of KCNA5 deficiency or dysfunction in AF remains unclear.
In the mammalian heart, the transmission of electrical activity is mediated by intercellular channels called junction channels, which aggregated into gap junctions between myocytes. Junction channels are composed of structurally related transmembrane proteins known as connexins (Cxs) that mainly connect the cytoplasm of adjacent cardiomyocytes. Among the connexins, connexin 43 (Cx43), connexin 40 (Cx40), and connexin 45 (Cx45) have been reported to be associated with the myocardial cell (Gros & Jongsma, 1996). Cx43 is the most abundant connexin and is found in all parts of the human organs, another major connexin, Cx40, is expressed selectively in atrial myocytes and mainly participated in the coordination of atria electrical activation (Gollob et al., 2006). What's more, Cx43 and Cx40 are reported to be involved in the occurrence and development of AF to varying degrees (Dhein, 2006; Zhang et al., 2017). AF changes in atrial function and structure during the progress of the disease and electrical remodeling normally occurs in the prophase of AF and may mediate subsequent atrial structural remodeling (Allessie et al., 2002). Recent studies have indicated that the expression of KCNA5 and Cx40 is synergistically regulated in the atrial myocytes (Zhang et al., 2017), nevertheless, there are few studies on the interaction and coexpression of KCNA5 with Cx43 and Cx40. Therefore, in this study we evaluated the expression levels and interactions of KCNA5, Cx43, and Cx40 proteins in patients with AF and explored the role of changes in KCNA5, Cx43, and Cx40 proteins in the pathological mechanism of AF, aiming to provide new strategies for the prevention and clinical treatment of AF.
2 MATERIALS AND METHODS
2.1 Patients and samples
A total of 10 patients with persistent AF underwent surgery in the cardiac surgery department of the Zhengzhou No. 7 People's Hospital was selected as the AF group and five normal sinus rhythm samples were taken from healthy heart donors who were not suitable for transplantation (no structural cardiac disease) as the Control group. About 200 mg of tissue in the left atrial appendage (LAA) was collected from both groups and was quickly frozen in liquid nitrogen and stored at −80°C until use. This study was approved by the Institutional Ethical Review Board at the Zhengzhou No. 7 People's Hospital and all the participants were enrolled with informed consent.
2.2 EXPERIMENTAL METHODS
2.2.1 Real-time polymerase chain reaction (PCR)
Total tissue RNA from frozen LAA tissues was extracted with Trizol reagent (15596026; Invitrogen) and 1 μg RNA was subjected to reverse transcription using PrimeScript™ RT Reagent Kit (RR047A; Takara) and incubated at 37°C for 15 min, then 85°C 5 s. For each sample, 2 μl of complementary DNA was amplified in a 20-μl PCR reaction system with TB Green® Premix Ex Taq™ II Kit (RR420A; Takara). The primer sequences of each gene are as follows: KCNA5 (encoding KCNA5 protein) (F: TGAGTTCAGGGATGAACGTG; R: GGTCTCCACGATGAAGAAGG) (Farah et al., 2020); GJA1 (encoding Cx43 protein) (F: GGAGTTCAATCACTTGGCGT; R: ACACCTTCCCTCCAGCAGTT) (Gemel et al., 2014); GJA5 (encoding Cx40 protein) (F: TCCTCGGAGTAGTGGTGAGATG; R: AAAGCTGAGGCTGCTGGTAAAG) (S. Li et al., 2017); glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (F: TCCTCTGACTTCAACAGCGA; R: GGGTCTTACTCCTTGGAGGC). PCR conditions were as follows, holding stage: 95°C for 30 s; cycling stage (40 cycles): 95°C for 5 s, 60°C for 30 s; melt curve stage: 65–95°C, increment 0.5°C for 5 s. And the relative expression levels of each gene in the two groups were calculated by the method.
2.2.2 Western blot analysis and immunoprecipitation (IP)
Total protein was extracted from LAA tissue by radioimmunoprecipitation assay (RIPA) solubilization buffer (Solarbio) and the protein concentration was measured by a BCA Protein Assay Kit. Then, 20μg of total protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes. After being incubated in 5% nonfat milk buffer for 1.5 h, PVDF membranes were probed overnight with primary antibodies of KCNA5 (1:1000, sc-377110; Santa Cruz), Cx43 (1:1000, sc-271837; Santa Cruz), Cx40 (1:1000, sc-365107; Santa Cruz) and GAPDH (1:1000, sc-47724; Santa Cruz) at 4°C. On the 2nd day, the membranes were incubated with a horseradish peroxidase-labeled secondary antibody (1:5000, A0216; Beyotime) for 1 h at 37°C. Peroxidase activity was viewed by enhanced chemiluminescence reagents using a chemiluminescence detection machine (Bio-Rad Laboratories, Inc.) ImageJ software (version V1.8.0.112; National Institutes of Health) was used for semiquantitative analysis of protein expression levels and GAPDH was used as the loading control.
For IP, total protein was extracted from LAA tissues by nondenatured lysis solution and protein A/G agarose beads (Santa Cruz) were added to collect supernatant and remove nonspecific binding proteins. Then primary antibodies of KCNA5 and corresponding immunoglobulin G (IgG) were added to the supernatant, respectively, and incubated at 4°C for 2 h to collect the immunocoprecipitate through centrifugation. The immunocoprecipitate was resuspended in RIPA buffer subsequently and 20 μg proteins were used for following western blot analysis.
2.2.3 Immunofluorescence and immunofluorescence colocation
The fresh frozen LAA sections (10 μm) were fixed by 4% paraformaldehyde and permeabilized with 1% Triton X-100 for 15 min, followed by blocking with 10% goat serum for 1.5 h at room temperature. Then sections were incubated overnight at 4°C with primary antibody (KCNA5, Cx43, Cx40 = 1:100). The next day, after washing with phosphate-buffered saline, samples were incubated with Cy3-conjugated secondary IgG antibody (1:500, A0521; Beyotime) or Alexa Fluor 488-conjugated secondary IgG antibody (1:500, A0423; Beyotime) for 1 h at room temperature. 4′,6-diamidino-2-phenylindole (C1002; Beyotime) was used to stain the nucleus for 5 min. Tissue sections were viewed using a fluorescent microscope (Olympus) and the fluorescence intensity was analyzed by ImageJ software (version V1.8.0.112; National Institutes of Health).
2.2.4 Statistical treatment
The measurement data were expressed as mean ± standard deviation (mean ± SD). The differences between the two groups were compared by Student's t test and SPSS 21.0 statistical software was used for statistical analysis. A p < .05 was considered statistically significant.
3 RESULTS
3.1 Analysis of KCNA5, GJA1, and GJA5 genes' messenger RNA (mRNA) levels
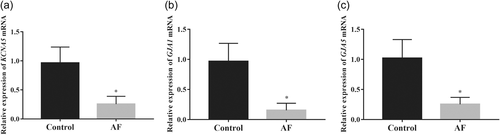
Real-time PCR was used to evaluate the relative expression of KCNA5, GJA1, and GJA5 mRNA levels in LAA of patients with AF and sinus rhythm and the results showed that levels of KCNA5 (Figure 1a), GJA1 (Figure 1b), and GJA5 (Figure 1c) were significantly decreased (p < .05) in the AF group compared with the control group with sinus rhythm.
3.2 Analysis of KCNA5, Cx43, and Cx40 protein levels
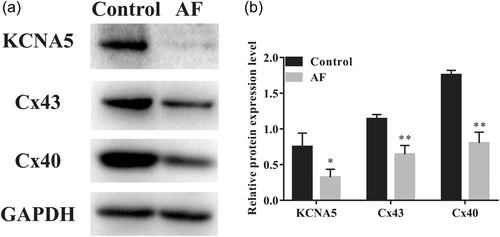
To determine whether overall KCNA5, Cx43, and Cx40 protein levels had changed in the AF group compared with the control group, we investigated these proteins of LAA tissues from AF patients and sinus rhythm patients by western blot analysis experiment. Western blot analysis results revealed that the relative expression levels of KCNA5, Cx43, and Cx40 proteins in the AF group were decreased as compared to the control group (Figure 2).
3.3 Immunofluorescence labeling of KCNA5, Cx43, and Cx40 proteins
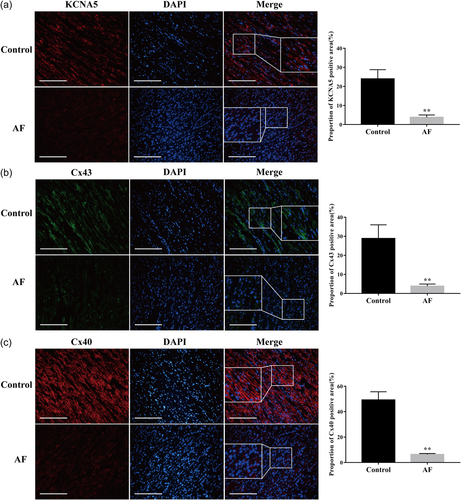
As immunofluorescence staining results showed, KCNA5, Cx43, and Cx40 proteins were mainly expressed in the cell membrane of human atrial myocyte and a small part was expressed on the cytoplasm (Figure 3). The fluorescence intensity was analyzed by ImageJ software and we observed the proportions of positive KCNA5, Cx43, and Cx40 proteins were significantly reduced in the AF group compared with the control group (p < .01).
3.4 Interactions between KCNA5 with Cx43 and Cx40 proteins
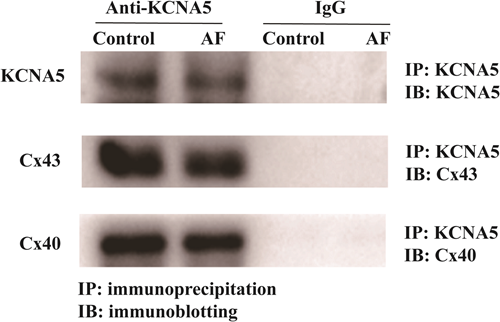
The interactions between KCNA5 with Cx43 and Cx40 proteins were analyzed by co-IP experiment. As IP results showed (Figure 4), with the precipitation of KCNA5, the coprecipitation of Cx43 was detected in both of control group and the AF group. Similar results were also found for the CX40 protein in LAA tissues of patients with AF and sinus rhythm, indicating the binding of KCNA5 with Cx43 and Cx40 protein in situ.
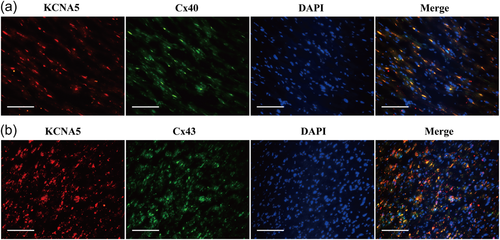
3.5 Colocalization of KCNA5 with Cx43 and Cx40 proteins
We then performed immunofluorescence double staining for KCNA5 and Cx43, KCNA5 and Cx40, respectively. The results of coimmunofluorescence staining revealed that the colocalization of KCNA5 and Cx43 were coexpressed in both plasma membrane and atrial myocytes. As Figure 5 showed the colocalization of KCNA5 and Cx40 were also tested in the membrane and cytoplasm of LAA tissues.
4 DISCUSSION
AF is the prevalent common arrhythmia in humans and may lead to palpitations, syncope, shortness of breath, chest pain, and even death. Nowadays, the effective prevention and treatment of AF have become a key area of cardiology research. However, the pathogenesis of AF has not been fully understood and the existing therapeutic strategies are not effective in the treatment of AF. The normal electrophysiological process of the heart is crucial to maintaining cardiac function. Studies have revealed that electrical and structural remodeling of atrial myocytes is one of the main pathological features of AF. Electrical remodeling and structural remodeling of the heart are regulated by changes in the expression levels and activity of ion channel membrane proteins and gap junction proteins (Allessie et al., 2002; Zhang et al., 2017). In this study, we collected LAA tissues from patients with sinus rhythm and AF and found that mRNA levels and protein levels of KCNA5, Cx43, and Cx40 were significantly downregulated in patients with AF compared with patients with normal sinus rhythm.
In atrial myocytes, changes in ion channels associated with AF play an important role in atrial electrical remodeling. At present, four voltage-gated potassium channels have been reported, namely, the Kv1 channel encoded by the KCNA gene, Kv2 channel encoded by KCNB, KCNC channel, and Kv4 channel encoded by KCND. Among them, Kv1.5 is a subtype of the Kv1 potassium channel (Coetzee et al., 1999). Kv1.5 is encoded by the KCNA5 gene and is mainly expressed in human atrial myocytes (Olson et al., 2006), which mediates the IKur in the atrium. IKur is a specific repolarization current in the atria and the reduced amplitude and function loss of IKur may lead to the occurrence of AF. Besides this, IKur has not been observed in ventricular myocytes (Varró et al., 2004). Therefore, specific inhibition of this channel by targeted drugs can prolong the repolarization time of the atrial current, thereby possibly terminating persistent AF (Varró et al., 2004). In addition, the pathogenic association between gene mutations and impaired function of Kv1.5 and susceptibility to AF has been verified in mouse models (McCauley et al., 2020; Olson et al., 2006; T. Yang et al., 2010). Our results showed that compared with patients with sinus rhythm, the mRNA transcription level and protein expression level of KCNA5 both declined in the LAA tissues of patients with AF, indicating that decreased expression of KCNA5 might be a risk factor for the occurrence of AF.
The disorder of gap junction protein arrangement is one of the characteristics of structural remodeling in AF and the gap junction in the heart is composed of about 40 gap junction channels. Two subtypes of gap junction protein are predominantly found in human atrial myocytes, namely, Cx43 and Cx40. The gap junction, as a direct signaling pathway between cells, plays an important role in maintaining normal physiological functions of the heart and arteries. In fact, gap junction protein regulates and stimulates the diffusion of electrical impulses that synchronously contract the heart and promotes the coordination of electrical activity in the artery wall cells. And once gap junction communication is impaired, normal tissue function cannot be maintained (Severs et al., 2001). In the cardiovascular system, gap junction mutations and protein expression defects are associated with congenital heart malformations (Dasgupta et al., 1999) and acquired cardiac rhythm disorders (Severs, 1999). It has been reported that ventricular ischemia leads to decreased Cx43 protein expression in canine hearts (Verheule et al., 2002). Other research has confirmed that the reduction of protein expression levels and redistribution of Cx40 and Cx43 contribute to the occurrence and persistence of AF in patients with rheumatic heart disease. Nevertheless, the decrease or increase of connexins expression is not always consistent in different animal models and in patients with different concomitant diseases (D. Q. Li et al., 2004; Wetzel et al., 2005). Therefore, the role of gap junction in the occurrence and development of AF is still unclear. Our research proved that compared with patients with sinus rhythm, the expression of Cx43 and Cx40 levels was reduced in the LAA tissues of patients with persistent AF. We speculate that the decrease of gap junction proteins in AF may affect the normal connection and conduction between atrial myocytes, leading to the potential transmission disorder, thus increasing the susceptibility to AF.
Atrial remodeling and electrical remodeling can mediate AF. Recent studies have demonstrated that the downregulation of Cx40 can inhibit KCNA5 expression in isolated cultured atrial myocytes. Conversely, silencing KCNA5 also inhibits Cx40 expression. This indicates that there may be a synergistic regulatory mechanism between Cx40 and KCNA5 (Zhang et al., 2017). In this study, it was discovered that KCNA5 could bind to Cx43 and Cx40, respectively, in the human LAA tissues and their mRNA levels and protein levels were maybe positively correlated. This suggests that electrical remodeling induced by ion channel alteration may lead to structural remodeling caused by gap junction protein changes during the occurrence of AF. Further, the combined effect of electrical remodeling and structural remodeling perhaps be one of the pathogenesis of AF. However, the specific regulatory mechanism still needs to be confirmed by further study.
In conclusion, our study confirmed that the expression levels of KCNA5, Cx43, and Cx40 in LAA tissues of AF patients were significantly decreased and KCNA5 with Cx43 and KCNA5 with Cx40 were both binding and coexpressing in protein expression levels. We hope all these results will be a foundation for further research on the pathogenesis of AF and it also provides a certain experimental basis for the exploration of new targeted antiarrhythmic drugs.
AUTHOR CONTRIBUTIONS
Zhikun Guo contributed to the conception of the study; Keke Wang performed the experiment and drafted the manuscript; Juntao Zhao performed the data analyses. All authors contributed to the article and approved the submitted version.
ACKNOWLEDGMENTS
This study was supported by grants from the Medical Science and Technology Project of Henan Province (LHGJ20191109).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.




