Hypoxia-induced SPOP attenuates the mobility of trophoblast cells through inhibition of the PI3K/AKT/GSK3β pathway
Abstract
Placental hypoxia has been implicated in pregnancy pathologies such as pre-eclampsia and intrauterine growth restriction. However, the underlying mechanism by which the trophoblasts respond to hypoxia remains unclear. Speckle‑type POZ protein (SPOP), an E3 ubiquitin ligase adapter, was previously reported to play important roles in various physiological and pathological processes. This study aims to investigate the expression and biological functions of SPOP after exposure to cobalt chloride (CoCl2)-mimicked hypoxia conditions using human trophoblast-derived choriocarcinoma cell lines and extravillous cytotrophoblast. These data showed that SPOP protein was directly induced by CoCl2-mimicked hypoxia and regulated by HIF-1α at the posttranscription level. CoCl2 treatment could dramatically influence the localization of SPOP in trophoblasts, especially the accumulation of SPOP into the nucleus. In addition, both CoCl2-mimicked hypoxia and induction of endogenous SPOP expression by lentivirus transfection attenuated the migration and invasion abilities of trophoblasts. Furthermore, we demonstrated that SPOP was involved in CoCl2-induced the inhibition of the PI3K/AKT/GSK3β pathway in placental trophoblasts. Taken together, these data indicate that accumulation of HIF-1α augments the expression of SPOP in trophoblasts, which impairs trophoblastic mobility by targeting the PI3K/AKT/GSK3β pathway. This potentially leads to insufficient uterine spiral artery remodeling and suboptimal placental perfusion, and thus the development of pregnancy-related complication.
Abbreviations
-
- ccRCC
-
- clear cell renal cell carcinoma
-
- CHX
-
- cycloheximide
-
- CoCl2
-
- cobalt chloride
-
- HIF-1α
-
- hypoxia-inducible factor 1α
-
- IUGR
-
- intrauterine growth restriction
-
- PE
-
- pre-eclampsia
-
- qRT-PCR
-
- quantitative real-time polymerase chain reaction
-
- SPOP
-
- speckle‑type POZ (pox virus and zinc finger protein) domain protein
-
- VHL
-
- von Hippel-Lindau
1 INTRODUCTION
During the first trimester of human pregnancy, extravillous cytotrophoblast cells invade basal decidua, which temporarily occlude the uterine spiral arteries and lead to placental hypoperfusion, thereby decrease nutrient and gas exchange between the mother and fetus or even placental hypoxia (Baumann et al., 2007; Zhou et al., 2016; Zhu et al., 2017). It is widely believed that placental hypoxia has been implicated in pregnancy-specific disorders, including pre-eclampsia (PE) and intrauterine growth restriction (IUGR; Caniggia & Winter, 2002; Gagnon, 2003; Granger et al., 2002). However, trophoblasts in response to hypoxia later in pregnancy have not been adequately explored.
One of the critical mediators that responds to oxygen deficits in multiple cells during physiological or pathological processes is hypoxia-inducible factor-1 (HIF-1; Patel et al., 2010; Semenza, 2013; Wang et al., 2017; Yang et al., 2018; Zhang et al., 2017). The HIF-1 serves as a basic helix-loop-helix transcription factor composed of two subunits, which are the oxygen-sensitive α-subunit (HIF-1α) and constitutively expressed β -subunit (HIF-1β, known as ARNT). Active HIF-1 complex could be formed when the remarkably increased HIF-1α expression dimerizes with HIF-1β under low oxygen conditions; conversely, under normoxic condition, HIF-1α became particularly unstable and could be rapidly degraded through the von Hippel-Lindau (VHL) E3 ligase complex-dependent ubiquitin-proteasome pathway (Chen et al., 2013; Katharina Flück, 2016). Previous studies have shown that HIF-1α was highly expressed in the early human placenta (at 7–10 weeks of gestation) and was decreased with advanced weeks of gestation in normal pregnancy (Ietta et al., 2006). Moreover, accumulating evidence suggested that constitutively expressing stabilized HIF-1α in pregnant mice could induce the pathogenesis of many pregnancy complications, including pre-eclampsia and IUGR (Tal et al., 2010).
SPOP is ubiquitous in various human tissues, which is known as the speckle-type POZ (pox virus and zinc finger protein) domain protein because of its patchy distribution in the nucleus (Nagai et al., 1997). SPOP protein consists of 374 amino acid residues, and its N-terminal and C-terminal contain a typical POZ/BTB domain and a MATH/TRAF domain, respectively. Studies have proved that the BTB domain could bind to the ubiquitin ligase cullin3 and act as a substrate adapter between Cul3 and a specific substrate (Lionel Pintard et al., 2003; Rory et al., 2003). Wild type SPOP has been found decreased expression in the majority of human cancers including prostate cancer, breast cancer, gastric cancer, pancreatic cancer, and endometrial carcinoma (Barbieri et al., 2012; Kim et al., 2013; Tan et al., 2019; Zeng et al., 2014; Zhang et al., 2015), which is considered a tumor suppressor. High expression levels of SPOP have been shown to act as a tumor promoter in other tumors such as clear cell renal cell carcinoma (ccRCC; Harb et al., 2018; Liu et al., 2009; Zhao et al., 2016). In addition, SPOP not only plays a role in the cancer pathogenesis and progression, but also affects some physiological processes to some extent. For example, Liu et al. (2016) observed that SPOP regulated endometrial stromal cell decidualization in mice and may be involved in embryonic implantation. SPOP could restrain the inflammatory activation of hematopoietic stem cells (HSCs) by ubiquitinating the innate signal transducer myeloid differentiation primary response protein 88 (MYD88), which is critical to restore homeostasis (Guillamot et al., 2019). Furthermore, a previous study has demonstrated a direct relationship between HIF-1α and SPOP levels in ccRCC (Li et al., 2014). Despite the fact that SPOP various roles in physiological and pathological conditions has been described, scarce data exist on the expression status and the regulatory mechanism of SPOP in placental trophoblast populations under normoxia and hypoxia conditions.
In this study, a cobalt chloride (CoCl2)-mimicked hypoxia model was used to elucidate the effects of HIF-1α on SPOP expression after exposure to hypoxia; SPOP-excessive/deficient trophoblast cells were used to investigate whether SPOP impacts migration and invasion abilities of trophoblasts, as well as to determine the relevance of SPOP to the subsequent development of pregnancy-related complication.
2 MATERIALS AND METHODS
2.1 Cell culture
A first-trimester extravillous cytotrophoblast cell line (HTR8-SVneo) was obtained from ATCC, and the human choriocarcinoma cell lines JAR and JEG3 cells were purchased from the Chinese Academy of Sciences. All cell lines were identified by the short tandem repeat (STR) genotyping. HTR-8/SVneo and JAR cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 and JEG3 cells was cultured in Dulbecco's modified Eagle's medium/F12 supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 ng/ml). All cell lines were incubated in a humidified atmosphere of 5% CO2 at 37°C. Cells were treated with hypoxia-mimetic agent CoCl2 (Sigma) at 150 µM to induce hypoxia and cycloheximide (CHX; ApexBio) at 25 µM to inhibit translation. For the exposure of cells to a low oxygen atmosphere (2%), trophoblasts were cultured in a hypoxic multi-incubator (Hypoxia Incubator Chamber) containing 2% O2, 5% CO2, and 93% N2 for 24 h.
2.2 RNA isolation, reverse transcription, and qRT-PCR
Total RNA was extracted from cultured cells with Trizol reagent (Takara). For real-time quantitative polymerase chain reaction (qRT-PCR), 1 μg of total RNA was reverse-transcribed into complementary DNA (cDNA) by using the Prime Script RT Reagent Kit (TakaRa). Synthesized cDNA was then amplified with a SYBR® Premix Ex Taq™ II (Perfect Real-Time) Kit (Takara). Melting curve for each target gene was generated to ensure PCR specificity. Relative abundance of messenger RNA (mRNA) was calculated by normalization to GAPDH mRNA expression. All the primers sequences used for amplification were listed in Table 1.
| Primer sequences for qRT-PCR | ||
|---|---|---|
| Gene | Product length | Sequence (5ʹ–> 3ʹ) |
| SPOP | 131 | GCCCTCTGCAGTAACCTGTC |
| GTCTCCAAGACATCCGAAGC | ||
| HIF-1α | 146 | TTACAGCAGCCAGACGATCA |
| GATTGCCCCAGCAGTCTACA | ||
| GAPDH | 59 | CGACCACTTTGTCAAGCTCA |
| CCCTGTTGCTGTAGCCAAAT |
- Abbreviation: qRT-PCR, real-time quantitative polymerase chain reaction.
2.3 Small interfering RNA (siRNA) transfection and lentivirus production and infection
JAR and JEG3 cells were transfected with a small interfering RNA (siRNA) specific for human HIF-1α (GenePharma) using Lipofectamine 2000 (Invitrogen) for 48 h. The HIF-1α-specific RNAi target sequence was as follows: siHIF-1α#1: 5′-CAG AAA UGG CCU UGU GAA ATT -3′; siHIF-1α#2: CAU GAG GAA AUG AGA GAA ATT. A nontargeting siRNA (5′-UUC UCC GAA CGU GUC ACG UTT-3′) was used as a negative control. For lentiviral infection, SPOP expression vector, a control vector, the shRNA targeting SPOP and a negative control vector were designed and constructed by Sunbio Medical Biotechnology Co., Ltd. The sequences of shRNA oligonucleotides against SPOP were as follows: shSPOP#1: CCGGCACAAGGCTATCTTAGCAGCTCTCGAGAGCTGCTAAGATAGCCTTGTGTTTTTTG; shSPOP#2: CCGGCACAGATCAAGGTAGTGAAATCTCGAGATTTCACTACCTTGATCTGTGTTTTTTG; shSPOP#3: CCGGGGAGAGTCAACGGGCATATAGCTCGAGATATGCCCGTTGACTCTCCATTTTTTTG. These vectors were transfected into JAR cells when cells fusion reached 50%. After 24 h, the culture medium was substituted with fresh medium. After 72 h of transfection, puromycin was added to the culture medium at a concentration of 5 mg/ml for 2 weeks to generate stable cell lines. Stable SPOP over-expressing or silenced cell lines were confirmed by western blot analysis and qRT-PCR.
2.4 Transwell assays
The cell invasion and migration assays were done in 24-well Transwell plates with or without pre-coated Matrigel. Briefly, 3 × 104 JAR cells infected with lentiviral stabilized overexpression or knockdown SPOP and the corresponding negative control cells were suspended in 200 μl serum free RPMI-1640 medium into the upper chamber. The upper chamber contained an 8-μm pore size membrane (BD Biosciences). In the lower chamber, 500 μl RPMI-1640 medium containing 20% FBS was added. After 48 h of incubation, JAR cells remaining in the upper chamber were softly removed by cotton swab scrubbing. The cells that successfully migrated through the membrane and invaded through the Matrigel were fixed in 4% paraformaldehyde for 20 min and stained with crystal violet dye for 15 min. The cell numbers were counted at six randomly selected views, and the average value was calculated.
2.5 Cell fractionation assays
Nucleoprotein and cytoplasmic protein were extracted according to Nucleoprotein and Cytoplasmic Protein Extraction Kit (Beyotime). The three trophoblasts were seeded in Petri dishes and cultured to 70% confluence. Cells were treated with hypoxia-mimetic agent CoCl2 (150 µM) for 0 h and 24 h, respectively. Centrifugation was used to collect the cells, absorbing the supernatant as much as possible, and leaving the cell precipitation. According to the instructions of the Extraction Kit, cytoplasmic protein and nuclear protein were extracted step by step, the extracted cytoplasmic and nuclear proteins were stored at −80℃ for future use.
2.6 Western blot analysis
The equivalent amounts of protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. Nonspecific binding sites of the membranes were blocked and incubated overnight with specific primary antibodies at 4°C. Then blots were probed with horseradish peroxidase-conjugated anti-rabbit (Proteintech, SA00001-2) or anti-mouse (Zhongshan Company, Beijing, ZB-2305) secondary antibodies for 1.5 h at room temperature. The primary antibodies used were as follows: HIF-1α (Santa Cruz, sc-13515), SPOP (Proteintech, 16750-1-AP), p-PI3K (phospho‑Tyr467, Cell Signaling Technology, 17366), t-PI3K (Cell Signaling Technology, 4292), p-AKT(phospho‑Tyr473, Cell Signaling Technology, 9271s), t-AKT (Proteintech, 10176-2-AP), p-GSK3β (phospho‑S9, Cell Signaling Technology), t-GSK3β (Cell Signaling Technology, 9315), GAPDH (Beyotime, AF5009), Histone H3 (Beyotime, AH433), and Tubulin (Beyotime, AT819). Immunoreactive bands were measured by enhanced chemiluminescence system (Millipore, WBKLS0100). Signals of the western blot bands were analyzed using the Image Lab program.
2.7 Immunofluorescence
Cells were seeded on chamber cover slips above 24 h and were fixed with 4% paraformaldehyde solution at room temperature for 20 min. After cells on cover slips were permeabilized and blocked in 3% BSA for 1 h, the cells were incubated with HIF-1α or SPOP antibodies (HIF-1α, Santa Cruz, sc-13515, 1:200; SPOP, Proteintech, 16750-1-AP, 1:200) at 4°C overnight, then Alexa Fluor 488- or 595-conjugated goat secondary antibodies (Beyotime) at 37°C for 1 h. For nuclear staining, cells were incubated with DAPI for 5 min. Cell images were captured with a confocal microscope (Nikon).
2.8 Statistical analyses
All statistical analyses were performed using the Graph Pad prism version 8.0 (Graph Pad Software). The statistical analyses were evaluated using a two-tailed Student's t-test and one-way analysis of variance. Data in this study were presented as mean ± SD. All results were from at least three independent experiments.
3 RESULTS
3.1 Activation of HIF-1α signaling in trophoblast-derived cell lines exposed to hypoxia
Two different human trophoblast-derived choriocarcinoma cell lines JAR and JEG3, and a first-trimester extravillous cytotrophoblast cell line HTR8-svneo have been widely used as in vitro models for human trophoblast studies. We characterized the expression and subcellular localization of HIF-1α in trophoblast-derived cell lines exposed to hypoxia-mimetic agent CoCl2 (150 µM) or cultured in a hypoxic multi-incubator (2% O2). Hypoxia-induced changes in protein expression and subcellular localization of HIF-1α were determined by western blot analysis and immunofluorescence staining. Western blot analysis of whole cell lysates showed that treatment of the three trophoblast-derived cells with CoCl2 significantly increased cellular HIF-1α protein compared to control cells (Figure 1a). Besides, in contrast to standard culture conditions (20% O2), exposure of trophoblast-derived cells to low oxygen levels (2% O2) also remarkably enhanced HIF-1α protein levels (Figures 1a and S1a).
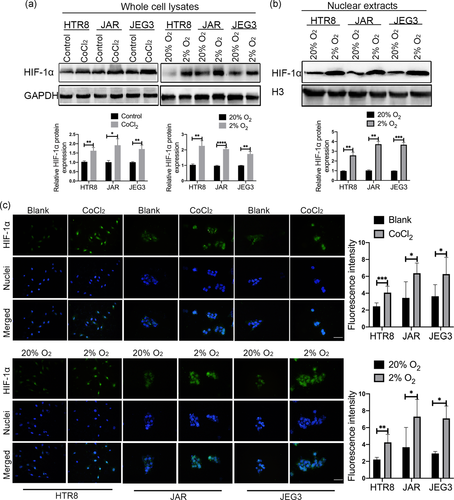
Next, we determined the subcellular localization of HIF-1α in the three trophoblasts, immunofluorescence analysis showed that HIF-1α was predominantly labeled in the nucleus of all three trophoblast-derived cells under CoCl2-mimicked hypoxia conditions or 2% O2 exposure (Figure 1c). As demonstrated by western blot analysis, HIF-1α protein was significantly increased in the nuclear extracts after low oxygen stimulations (Figure 1b). In summary, these results demonstrated that HIF-1α is activated by hypoxic stimulation in the three trophoblast-derived cell lines.
3.2 SPOP expression is induced by CoCl2-mimicked hypoxia
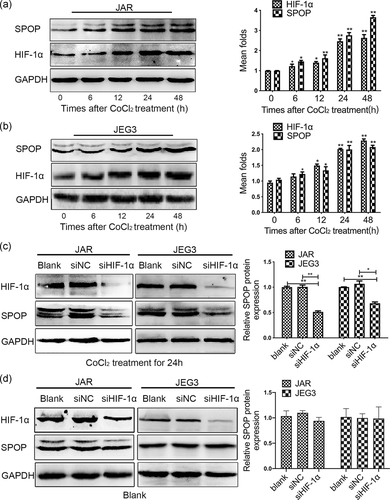
Due to the limitation of experimental conditions, trophoblasts were subjected to CoCl2 treatment in the following experiments to establish an in vitro hypoxia model, rather than exposed to 2% O2 in a multigas incubator. As shown in Figure 2a,b, human choriocarcinoma cell lines received CoCl2 treatment resulted in significantly improved expression of HIF-1α, and a gradual increase of SPOP level both at the protein levels. Importantly, we constructed two HIF-1α siRNA vectors and selected the siHIF-1α#2 sequence with the most obvious knockdown effect for subsequent experiments (Figure S1b). Compared with control cells, transfection with siRNA against HIF-1α remarkably restrained the expression of SPOP in JAR and JEG3 cells following CoCl2 treatment (Figure 2c). However, no significant changes in SPOP expression were observed between HIF-1α siRNA and control groups in the absence of CoCl2 (Figure 2d), indicating that the basal level of SPOP production is independent of HIF-1α.
3.3 SPOP is stabilized by HIF-1α at posttranscription
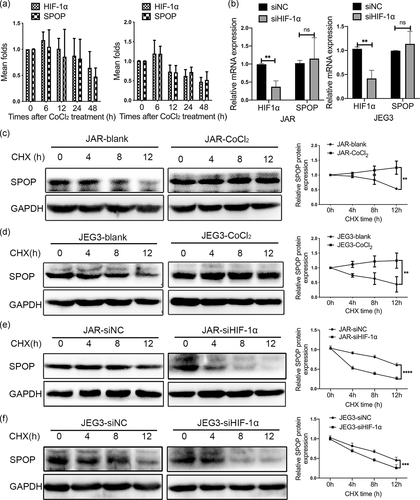
The protein expression of SPOP was positively correlated with the expression of HIF-1α in trophoblast-derived cell lines after CoCl2 treatment. However, the mRNA level of SPOP had no obvious change neither by CoCl2 treatment (Figure 3a) nor HIF-1α knockdown (Figure 3b), implying that the effect of HIF-1α on SPOP protein levels was not mediated through the increase of SPOP mRNA expression, and SPOP might be regulated at the posttranscriptional levels. In addition, the stability of SPOP protein was significantly affected by protein synthesis inhibitor CHX treatment, the treatment of CoCl2 remarkably extended the half-life of SPOP protein in JAR and JEG3 cells (Figure 3c,d). Conversely, the stability of SPOP protein was further reduced by CHX treatment after HIF-1α knockdown compared with negative control cells (Figure 3e,f). These results suggested that HIF-1α stabilized the expression of SPOP at posttranscription level.
3.4 Hypoxia could drive SPOP accumulation in the nucleus
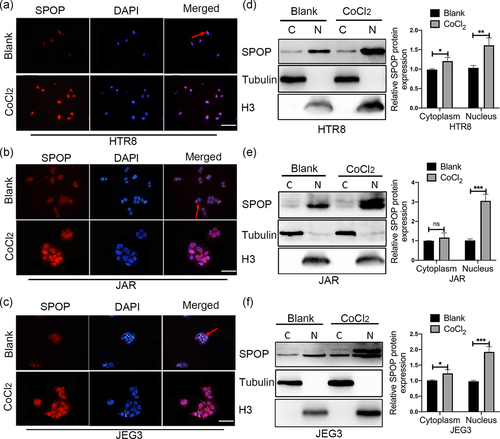
SPOP was first identified as a nucleoprotein (Nagai et al., 1997). Previous studies have shown that the subcellular localization patterns of some proteins are differentially altered in response to hypoxia (Wang et al., 2018; Yunes-Medina et al., 2017). Considering the different functions of SPOP in cytoplasm and nucleus, we examined the subcellular localization pattern of endogenous SPOP in trophoblasts. Immunofluorescence analysis revealed that SPOP was localized exclusively in the nucleus in the majority of the trophoblasts (Figure 4a–c). However, in a small number of trophoblasts, SPOP was labeled in both the nucleus and cytoplasm. CoCl2 treatment markedly enhanced the fluorescence intensity of SPOP in the nucleus (Figure 4a–c). In addition, SPOP accumulation in the nucleus under CoCl2-mimicked hypoxic conditions was further verified by cell fractionation assays (Figure 4d–f). Taken together, these findings suggested that CoCl2 treatment could dramatically influence the localization of SPOP in trophoblast-derived cell lines, especially the accumulation of SPOP into the nucleus.
3.5 SPOP regulates migration and invasion of trophoblasts through the PI3K/AKT/GSK3β signaling pathway
To determine the biological roles of SPOP in the development and progression of trophoblasts, we stably overexpressed and knocked down SPOP in JAR cells using a lentivirus-based system. Levels of SPOP in these resultant cell lines were confirmed by western blot analysis and qRT-PCR (Figure 5a,b). Three SPOP interference vectors were constructed, of which shSPOP#1 was the most effective for subsequent functional experiments (Figure S1c). Functionally, transwell assays revealed that forced expression of SPOP obviously inhibited the migration and invasion abilities of JAR cells, whereas knockdown of SPOP significantly increased the number of migrated and invaded cells (Figure 5c,d).
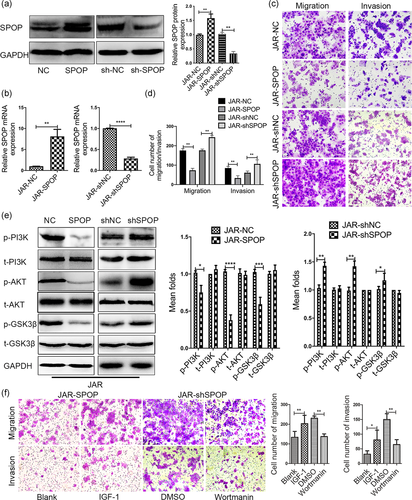
To further investigate whether PI3K/AKT/GSK3β signaling pathway is involved in the regulation of trophoblasts by SPOP, we identified the expression levels of associated proteins. As seen in Figure 5e, JAR cells with SPOP overexpression resulted in decreased levels of phosphorylated AKT and GSK-3β, whereas the decrease of AKT and GSK-3β phosphorylation were reversed after SPOP depletion. These data indicate that SPOP restrains the activation of the PI3K/AKT/GSK3β pathway in JAR cells. Next, we treated JAR cells overexpressing SPOP with insulin-like growth factor 1 (IGF-1), which is an activator of the PI3K/AKT pathway. We found that IGF-1 treatment at least partially rescued the SPOP-induced inhibition of JAR cell migration and invasion. Conversely, the suppression of the PI3K/AKT pathway by wortmanin abrogated the effects of SPOP loss on cell migration and invasion (Figure 5f). Thus, our results imply that the effect of SPOP on trophoblasts mobility may be mediated by regulating PI3K/AKT/GSK3β signaling.
3.6 SPOP mediates CoCl2-inducible PI3K/AKT/GSK3β pathway inactivation in placental trophoblasts
We first investigated whether CoCl2 incubation induced the activation of the PI3K/AKT pathway in trophoblast cell lines, JAR cells were subjected with CoCl2-mimicked hypoxia treatment for 6 h or 24 h. The results showed that CoCl2 treatment dramatically attenuated the expressions of phospho-Ser-473 AKT and phospho-Ser-9 GSK-3β in comparison to control cells, which was more marked at 24 h than at 6 h. Especially, early hypoxia (6 h) stimulation caused a slightly elevation in phospho-Ser-9 GSK-3β expression. Moreover, to verify that the effect of CoCl2 incubation on AKT and GSK-3β phosphorylation was not due to a change in the total amount of AKT and GSK-3β protein, western blot for total protein were performed. A 6 and 24 h incubation time of CoCl2 did not alter the total protein level of GSK-3β, while longer CoCl2 treatment (24 h) caused a significant decrease in total AKT protein expression compared to the control group (Figure 6a).
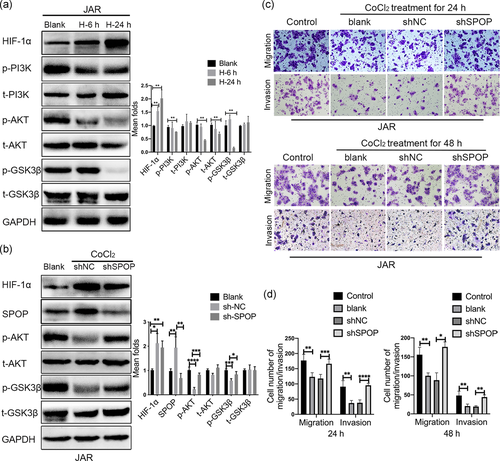
To examine whether SPOP is involved in CoCl2-induced the inhibition of PI3K/AKT/GSK3β pathway in placental trophoblasts. We simultaneously knockdown SPOP in JAR cells under CoCl2-mimicked hypoxia conditions, the results showed that SPOP knockdown reversed the downregulation of AKT and GSK-3β phosphorylation levels in CoCl2 treated cells (Figure 6b), implying that inhibition of SPOP expression at least partially alleviated the dephosphorylation of AKT and GSK-3β induced by CoCl2. Furthermore, CoCl2 treatment of JAR cells for 24 h or 48 h significantly suppressed the migration and invasion, this suppression was blocked in the presence of SPOP depletion (Figure 6c,d). Altogether, these results suggested that CoCl2-inducible upregulation of SPOP expression compromises trophoblastic invasion and migration through the PI3K/AKT/GSK3β signaling pathway.
4 DISCUSSION
SPOP has been extensively studied in tumorigenesis and is thought to play crucial roles in cancer pathogenesis and progression via complex mechanism (Duan & Pagano, 2015; Hjorth-Jensen et al., 2018; Zhu et al., 2015). Since placental trophoblasts in early pregnancy have shown many common characteristics to malignant cells, making them ideal for monitoring molecular changes taking place during the acquisition of a migrating/invasive phenotype (Ferretti et al., 2007). Accumulating evidence has suggested that SPOP play an important role in maintaining normal uterine function, including embryo implantation and endometrial stromal cell decidualization in mice (Hai et al., 2018; Liu et al., 2016). In this study, considering that the placenta is often under a hypoxic microenvironment in both the first trimester pregnancy and some placental specific pathological conditions, we established the hypoxic trophoblast model by the hypoxia-inducing chemical agents CoCl2 and found that CoCl2 incubation induced SPOP expression. Besides, we revealed for the first time that CoCl2-inducible upregulation of SPOP expression was involved in the suppression of the PI3K/AKT/GSK3β pathway associated with hypoxia. The inactivation of the PI3K/AKT/GSK3β pathway impaired the migration and invasion of trophoblasts, and thus contributing to defective placentation and fetal growth restriction, which both are associated with pre-eclampsia.
Placental hypoxia is widely accepted to play a critical role in placental pathologies, especially pre-eclampsia (Young et al., 2010). HIF-1α is a key mediator in response to hypoxia stimulation and contributes to placental differentiation, growth and function. Placental trophoblasts such as human extra-chorionic trophoblast (HTR8-SVneo) and immortalized human choriocarcinoma cell lines (JAR and JEG3) are specialized epithelial cells with functions and phenotypes very close to those of trophoblast cells in vivo, known to be sensitive to changes in oxygen content early in placental development. Previous in vitro studies have shown that accumulation of HIF-1α in early gestation could impair extravillous trophoblastic invasion and migration through inhibition of the SRC-3/AKT/mTOR axis (He et al., 2019). Several pro- and anti-invasion factors expressed in placental trophoblasts or maternal decidua are HIF-1α target genes (Fang et al., 2017; Ma et al., 2019; Sasagawa et al., 2018; Zhu et al., 2017). Moreover, hypoxia directly regulates human extravillous trophoblast differentiation through HIF-dependent modulation of various cell adhesion and morphology-related pathways (Wakeland et al., 2017). This series of findings indicate that trophoblasts migration/invasion can be modulated by HIF-1α. In our studies using trophoblast cells, we demonstrated that SPOP was activated following exposure to CoCl2 treatment, and both SPOP overexpression and CoCl2-mimicked hypoxia compromised the migration and invasion capacities of trophoblasts, suggesting that increased SPOP production may be one of the reasons for the suppression of cells migration and invasion caused by CoCl2-mimicked hypoxia.
Moreover, we demonstrated that CoCl2-induced SPOP could be stabilized by HIF-1α at the posttranscriptional level. HIF-1α, as an important transcription factor, we first detected whether HIF-1α can induce the expression of SPOP through transcriptional activation in trophoblasts, but contrary to our expectation, CoCl2 stimulation cannot affect the level of SPOP mRNA; indicating that the effect of HIF-1α on SPOP protein levels is not mediated at the transcriptional level. Posttranscriptional regulation of protein is the main mechanism affecting protein expression. There are many factors that affect the processes of posttranscriptional regulation, such as microRNAs (miRNAs), a cluster of noncoding RNAs that are important for regulating gene expression at the posttranscriptional level through binding to 3′-untranslated region of target genes, thereby the expression level of mRNA may remain unchanged, but resulting in the inhibition of protein translation and stability (Flynt & Lai, 2008). In addition, posttranslational modification of proteins, including phosphorylation, ubiquitination, glycosylation, acetylation, and so forth, can affect protein levels. Especially in ubiquitination processes, which affects protein degradation process and protein content through the regulation of ubiquitination proteasome system, while mRNA level may not change (Cai et al., 2019; Jin et al., 2020). Considering the complexity and diversity of the effect of hypoxic environment on organisms, although HIF-1α usually functions as a transcription factor through directly binding with target genes, it does not exclude the possibility of posttranscriptionally regulating SPOP protein by influencing the expression of other intermediates, such as some miRNAs, phosphorylase, ubiquitinase, acetylase, and so forth. However, the specific regulatory mechanism of HIF-1α on SPOP expression needs to be further studied.
Concerning the regulatory mechanism of trophoblasts exposed to hypoxia, we explored one critical PI3K/AKT signaling pathway about SPOP function in trophoblasts, which is involved in the multiple biological behaviors of trophoblasts, such as proliferation, migration, and invasion (Changwon Yang et al., 2018; Chen et al., 2019; Wang et al., 2019). Previous studies have showed that increased cytoplasmic SPOP under hypoxia promoted ccRCC tumorigenesis by modulating multiple pathways, including PTEN/AKT pathway and DUSP7/ERK pathway (Li et al., 2014). Different from their study, our data showed that increased nuclear SPOP under hypoxia inhibited the invasion and migration of trophoblasts by inactivating PI3K/AKT signaling pathway. The increased SPOP protein in different cells after hypoxic treatment was localized in cytoplasm or nucleus respectively. Based on the previous research works, SPOP located in the nucleus of the normal cells plays the role of tumor suppressor (Boohyeong Byun, 2007), while the accumulation of SPOP in the cytoplasm of the tumor cells plays the role of tumor promoter (Li et al., 2014; Liu et al., 2009). Consistent with this notion, our experiments elucidated that CoCl2 treatment could primarily promote the accumulation of SPOP to nucleus, implying that CoCl2-induced upregulation of SPOP suppressed biological behaviors of human placental trophoblasts.
In summary, we demonstrate for the first time that both upregulation of SPOP induced by CoCl2 treatment and endogenous SPOP overexpression in trophoblasts result in the suppression of trophoblasts migration and invasion through the inhibition of the PI3K/AKT/GSK3β pathway. These findings may contribute not only to understanding the molecular mechanism of increased SPOP production in human placental pathologies associated with hypoxia, but also to developing a novel potential target for therapeutic intervention.
ACKNOWLEDGMENT
The present study was supported by the National Natural Science Foundation of China (grant no. 81100443).
CONFLICTS INTEREST
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Open Research
DATA AVAILABILITY STATEMENT
Data openly available in a public repository that issues datasets with DOIs.




