RICK regulates the odontogenic differentiation of dental pulp stem cells through activation of TNF-α via the ERK and not through NF-κB signaling pathway
Abstract
Dental pulp stem cells (DPSCs) are capable of both self-renewal and multilineage differentiation, which play a positive role in dentinogenesis. Studies have shown that tumor necrosis factor-α (TNF-α) is involved in the differentiation of DPSCs under pro-inflammatory stimuli, but the mechanism of action of TNF-α is unknown. Rip-like interacting caspase-like apoptosis-regulatory protein kinase (RICK) is a biomarker of an early inflammatory response that plays a key role in modulating cell differentiation, but the role of RICK in DPSCs is still unclear. In this study, we identified that RICK regulates TNF-α-mediated odontogenic differentiation of DPSCs via the ERK signaling pathway. The expression of the biomarkers of odontogenic differentiation dental matrix protein-1 (DMP-1), dentin sialophosphoprotein (DSPP), biomarkers of odontogenic differentiation, increased in low concentration (1–10 ng/ml) of TNF-α and decreased in high concentration (50–100 ng/ml). Odontogenic differentiation increased over time in the odontogenic differentiation medium. In the presence of 10 ng/L TNF-α, the expression of RICK increased gradually over time, along with odontogenic differentiation. Genetic silencing of RICK expression reduced the expression of odontogenic markers DMP-1 and DSPP. The ERK, but not the NF-κB signaling pathway, was activated during the odontogenic differentiation of DPSCs. ERK signaling modulators decreased when RICK expression was inhibited. PD98059, an ERK inhibitor, blocked the odontogenic differentiation of DPSCs induced by TNF-α. These results provide a further theoretical and experimental basis for the potential use of RICK in targeted therapy for dentin regeneration.
Abbreviations
-
- ALP
-
- alkaline phosphatase
-
- BMMSC
-
- bone marrow mesenchymal stem cell
-
- CARD
-
- caspase-recruitment domain
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- DMP-1
-
- dental matrix protein-1
-
- DPSC
-
- dental pulp stem cell
-
- DSPP
-
- dentin sialophosphoprotein
-
- FBS
-
- fetal bovine serum
-
- GAPDH
-
- glyceraldehyde-3-phosphate dehydrogenase
-
- IL-1
-
- interleukin-1
-
- MAPK
-
- mitogen-activated protein kinase
-
- MDP
-
- muramyl dipeptide
-
- MSC
-
- mesenchymal stem cell
-
- NF-κB
-
- nuclear factor-κB
-
- ODI
-
- odontogenic differentiation medium
-
- PD
-
- PD98059
-
- RICK
-
- Rip-like interacting caspase-like apoptosis-regulatory protein kinase
-
- RT-PCR
-
- reverse-transcription polymerase chain reaction
-
- SCAP
-
- stem cells originating from the apical papilla
-
- SDS
-
- sodium dodecyl sulfate
-
- SDS-PAGE
-
- SDS-polyacrylamide gel electrophoresis
-
- SHED
-
- stem cells originating from exfoliated deciduous teeth
-
- TLR
-
- toll-like receptor
-
- TNF-α
-
- tumor necrosis factor-α
1 INTRODUCTION
Dentin plays a significant role in providing physical protection for the dental pulp and support of the enamel structure. With the increase of age and in the presence of pathological stimulation, the structure of dentin undergoes a series of changes. The regulation of odontoblast differentiation as well as the formation of secondary dentin are crucial for dentinogenesis during the repair of tooth hard tissue (Liao et al., 2019). Human dental pulp-derived stem cells (DPSCs), a unique group of undifferentiated mesenchymal stem cells (MSCs) with the ability of self-renewal and multiple differentiation potentials, are a crucial element for reparative dentin (Aydin & Sahin, 2019; D. Wang et al., 2019; X. Yang et al., 2018). Furthermore, as medical waste, the pulp tissues in extracted teeth can be acquired easily without serious ethical and technical problems. The features of directional migration of DPSCs to damaged sites and their proliferation and differentiation to odontoblasts have been demonstrated, as well as their ability to form the tertiary dentin (Yu et al., 2015). Several signaling pathways involved in odontogenic differentiation appear to be consistent with both the development and repair of DPSCs, but the signaling environment after an injury is considered to be more complex and variable than that during development (He et al., 2015; Yuan et al., 2019). The diseased pulps are often exposed to an inflammatory microenvironment, but the unknown molecular mechanism of DPSCs in dentinogenesis limits their effective application in dentin tissue engineering. Hence, the repair of defective dentin tissue is still a significant challenge in the clinic (X. Wang et al., 2014).
The inflammatory response around the dental pulp tissue often takes place after dental caries or traumas and is characterized by the accumulation of inflammatory cells (Lima et al., 2018; M. Wang et al., 2017). Inflammatory cells release various inflammatory cytokines, including tumor necrosis factor-α (TNF-α). TNF-α, a marker of early inflammation, has extensive biological activity and participates in many inflammatory diseases, including inflammatory bowel diseases and neuroinflammation (Choy & Panayi, 2001; Kany et al., 2019; Kjeldsen et al., 1993). TNF-α has been shown to induce odontogenic differentiation of MSCs through modulation of the immune response (C. Li et al., 2016). Several studies have investigated the effect of TNF-α on osteogenetic differentiation of bone marrow mesenchymal stem cells (BMMSCs; Y. W. Wang et al., 2015). However, the mechanism involved in dentin repair induced by TNF-α has not been described.
Rip-like interacting caspase-like apoptosis-regulatory protein kinase (RICK), also known as RIP2 or CARDIAK, is a caspase-recruitment domain (CARD)-containing, putative serine/threonine protein kinase (Dufner & Mak, 2006; Yao, 2013). The expression of RICK is regulated at the transcriptional level in a cell-specific and timeline-dependent manner, which reflects the situation in the early inflammatory response (Watanabe et al., 2019). Under cell-specific activation stimuli, such as bacterial peptidoglycan and lipopolysaccharide, the expression of RICK increases (Lu et al., 2018). Furthermore, RICK has been shown to regulate the differentiation of cells, including human umbilical cord blood-derived mesenchymal stem cells, skeletal myoblasts, normal myoblasts, and rhabdomyosarcoma cells (H. S. Kim et al., 2013; Mueck et al., 2011).
Several studies showed that RICK activates pro-inflammatory signaling pathways, including nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK; Kapplusch et al., 2019; S. Li et al., 2019). MAPK signaling pathway family contains phosphorylation of ERK, JNK, and p38 (Braicu et al., 2019). Recent studies also found that MAPKs, particularly ERKs, could induce odontogenic differentiation of human DPSCs (Ge et al., 2020). A previous study has shown that TNF-α can regulate the osteogenic differentiation of umbilical cord-derived MSCs by triggering the NF-κB signaling pathway (Marupanthorn et al., 2015). However, whether and how RICK regulates odontogenic differentiation of DPSCs still remains unknown.
In this study, we investigated the role of RICK in the odontogenic differentiation of DPSCs after exposure to different concentrations of TNF-α.
2 MATERIALS AND METHODS
2.1 Cell cultures
The human DPSCs were derived from impacted fully developed healthy third molars, which were surgically removed from healthy young patients in the clinic with the indication of ectopia (18–23 years of age, n = 7), and healthy premolars extraction from orthodontic patients (13–14, years of age, n = 2). Informed consent was obtained from each patient, and the study was approved by the institutional Medical Ethics. Pulp tissue was isolated from the crown and digested in phosphate-buffered saline (PBS) containing 3 mg/ml collagenase type I and 4 mg/ml dispase for 1 h at 37°C. Single-cell suspensions were obtained by passing the digested tissues through a 70-mm cell strainer (BD Falcon). Cell suspensions of dental pulp were then seeded into 10-cm culture dishes and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Gibco-BRL; Life Technologies Inc.), 100 U/ml penicillin, and 100 mg/ml streptomycin at 37°C in 5% CO2. Cells were passaged at the ratio of 1:3 when they reached 85%–90% confluence. Cells between the third to fifth passages were used in the following experiments.
2.2 Odontogenic differentiation
Third-passage DPSCs (2 × 104 cells/dish) were seeded in 35-mm culture dishes (Costar) and cultured with odontogenic differentiation medium (ODI) consisting of α-MEM supplemented with 10% FBS, 50 mg/ml α-ascorbic acid, 10 mmol/L β-glycerophosphate, 10 nmol/L dexamethasone (Sigma-Aldrich) and 0.3 mg/ml glutamine, 100 units/ml penicillin G, 100 mg/ml streptomycin, respectively, for 0, 3, 7, and 14 days with or without TNF-α (1, 10, 50, 100 ng/ml), replacing the medium every 2 days. TNF-α was added every 3 days. At the indicated times, the cells were washed with PBS and fixed with 4% paraformaldehyde for 30 min before staining with Alizarin red S (Sigma) and alkaline phosphatase (JianCheng) at room temperature.
DPSCs were plated at a density of 2 × 104 cells/cm2 and cultured in an odontogenic medium with or without TNF-α (10 ng/ml) for 3 days. For inactivation of ERK signaling pathway, DPSCs were supplemented with 50 mM PD98059 (PD, a potent and selective inhibitor of MEK1, which is a kinase upstream of ERK; Sigma) for 3 days.
2.3 Alizarin red S and ALP staining
Third-passage DPSCs were plated to six-well plates (Falcon) and cultured in different dishes supplemented with ODI. DPSCs were fixed with 4% PFA for 1 h and washed with PBS. Cells were then stained with 40 mmol/L alizarin red S (pH 4.2) for 10 min under conditions of gentle agitation. After decoloration and air-drying, culture plates were evaluated by light microscopy using an inverted microscope. To quantify the red dye, the stain was solubilized by shaking with 10% cetylpyridinum chloride for 20 min and the absorbance was measured at a wavelength of 570 nm. The alkaline phosphatase (JianCheng) staining was used to estimate the degree of extracellular matrix calcification. DPSCs were subjected to alkaline phosphatase (ALP) staining using the ALP assay kit according to the manufacturer's instructions.
2.4 Western blot analysis
Western blot analysis was performed according to a previous study (Tsugawa et al., 2003). In brief, cells were washed twice with ice-cold PBS and lysed in buffer containing 50 mM Tris (PBST), 150 mM NaCl, 2% sodium dodecyl sulfate (SDS), and a protease inhibitor mixture. After centrifugation at 12,000 rpm for 12 min, protein concentrations were determined using the Bradford Microassay (Bio-Rad). The resulting supernatant (50 mg of protein) was subjected to 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were then transferred onto polyvinylidene difluoride membranes (Amersham Biosciences, Amersham, UK). The samples were incubated with the primary antibodies (1:400) overnight at 4°C and the HRP-conjugated secondary antibodies (1:1000) for 2 h. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control for the cytoplasmic and nuclear proteins. Concomitantly, the primary antibodies (Santa Cruz) used in this study were as follows: GAPDH (anti-mouse), DMP-1 (anti-mouse), DSPP (anti-mouse), ALP (anti-mouse), RICK (anti-mouse), IκBα (anti-rabbit), p-IκBα (anti-mouse), ERK (anti-mouse), p-ERK (anti-mouse), JNK (anti-mouse), p-JNK (anti-mouse), p38 (anti-mouse), p-P38 (anti-mouse). Western blot band area/intensity was measured as previously described (Huang et al., 2019). Bands area/intensity for all proteins was measured by Image J software (Wayne Rasband, National Institutes of Health).
2.5 Immunofluorescent staining
DPSCs were fixed with 4% PFA for 1 h, washed with PBST, and blocked for 30 min in PBST supplemented with 10% FBS. Cells were then incubated with one of the following primary antibodies (1:100; Santa Cruz) in the same solution overnight at 4°C Vimentin (anti-mouse), p65 (anti-rabbit), STRO-1 (anti-mouse), and CK-14 (anti-mouse). Cells were washed and incubated in secondary antibodies for 2 h at room temperature. Nuclei were stained with DAPI (1:800; Sigma). The cells were finally examined by a Leica fluorescence microscope (Germany).
2.6 RICK RNA silencing
The sequences of the sense and antisense strands of the human RICK siRNA were as follows: 5′-AGAAUAUCCUAUUGGACAATT-3′ (sense), 5′-UUGUCCAAUAGGAUAUUCUTT-3′ (antisense). The siRNA was commercially synthesized (Genepharma). For controls, scrambled RNA oligonucleotides were used. For each well, 33.3 nM of each were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After incubation for 6 h, the medium was replaced with DMEM containing 10% FBS. Transfected cells were used for the subsequent experiments 48 h after transfection.
2.7 Reverse-transcription polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from DPSCs using the TRIzol reagent (Life Technologies, Gaithersburg, MD) according to the manufacturer's instructions. RNA (2 μg) was reverse-transcribed into first-strand cDNA (Gibco BRL). PCR amplification was performed using the following primer sets: β-actin, F, 5ʹ-CGTTGACATCCGTAAAAGAC-3ʹ; R, 5ʹ-TGGAAGGTGGACAGTGAG-3ʹ. RICK, F, 5ʹ-CCATCCCGTACCACAAGCTC-3ʹ; R, 5ʹ-GCAGGATGCGGAATCTCAAT-3ʹ. p65, F, 5ʹ-CAAGCGAGGAGGGGACGTG-3ʹ; R, 5ʹ-CCCCCAGAGCCTCCACCC-3ʹ. DSPP, F, 5ʹ-GGAGCCACAAACAGAAGCAACA-3ʹ; R, 5ʹ-TGGACAACAGCGACATCCTCAT-3ʹ. DMP-1, F, 5ʹ-CAGGAAGAGGTGGTGAGTGAGT-3ʹ; R, 5ʹ-TGGATTCGCTGTCTGCTTGCT-3ʹ. GAPDH, F, 5ʹ-CCAGAACATCATCCCTGCCTCT-3ʹ; R, 5ʹ-GACGCCTGCTTCACCACCTT-3ʹ. PCR reactions were carried out using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems) with the following program: 95°C for 15 min, 94°C for 15 s, and 57°C for 30 s for 40 cycles. The mRNA levels of target genes were calculated after normalization to β-actin mRNA.
2.8 Statistical analysis
Data are shown as mean ± SEM from at least three independent experiments, each performed with triplicate samples. The differences between control and the experimental groups were determined by using one-way analysis of variance (ANOVA), multi-way ANOVA and Student's t test for post hoc comparisons. All statistical analyses were performed using SPSS 20.0 software. *p < .05 was considered statistically significant.
3 RESULTS
3.1 TNF-α influences the odontogenic differentiation of DSPCs
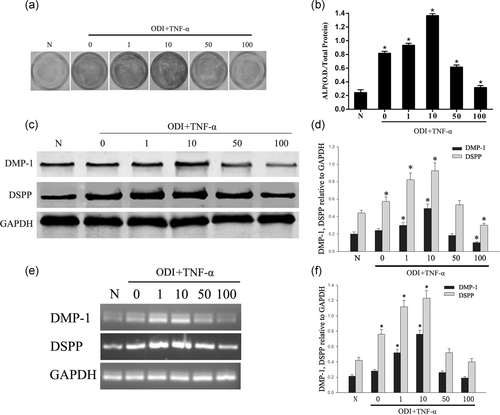
DPSCs were treated with different concentrations of TNF-α in ODI to examine the reparative responses of DPSCs to inflammatory stimuli. After 7 days of incubation, the activity of ALP was inhibited by TNF-α at high concentrations (50, 100 ng/ml) while the odontogenic potential of DPSCs was promoted at low concentrations (1, 10 ng/ml), especially at 10 ng/ml (Figure 1a,b; *p < .05). The expression of the mineralization-associated proteins DMP-1 and DSPP were tested during the 7 days of incubation. Western blot analysis showed that high concentrations (50, 100 ng/ml) of TNF-α downregulated the expressions of DMP-1 and DSPP in DPSCs compared with the control group (0 ng/ml TNF-α), while in low concentrations (1, 10 ng/ml) of TNF-α the expressions of DMP-1 and DSPP increased significantly, especially in 10 ng/ml (Figure 1c,d; *p < .05). RT-PCR analysis showed that low concentrations (1, 10 ng/ml) of TNF-α upregulated the expressions of DMP-1 and DSPP in DPSCs compared with the control group (0 ng/ml TNF-α), especially in 10 ng/ml (Figure 1e,f; *p < .05).
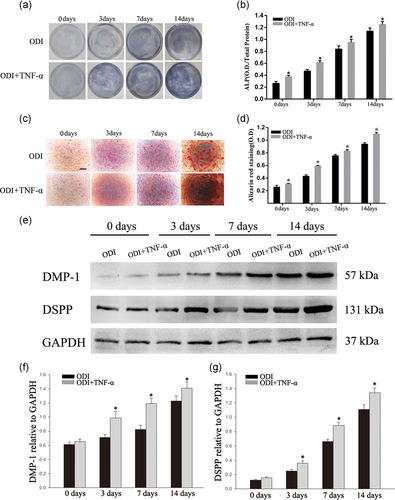
To investigate the effect of TNF-α on odontogenic differentiation, DPSCs were stimulated with TNF-α (10 ng/ml) for 0, 3, 7, and 14 days. ALP staining showed that TNF-α accelerated odontogenic differentiation of DPSCs time-dependently (Figure 2a,b; *p < .05). The results of Alizarin red S staining also indicated that TNF-α (10 ng/ml) promoted the formation of mineralized nodules (Figure 2c,d; *p < .05). The western blot analysis also showed that TNF-α (10 ng/ml) promoted the expressions of DMP-1 and DSPP at 3 days of odontogenic differentiation (Figure 2e–g; *p < .05).
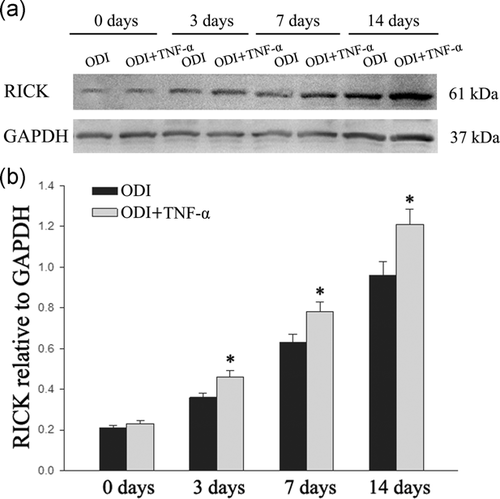
3.2 The expression of RICK increased in the odontogenic differentiation of DPSCs
The expression of RICK has also been tested to investigate the effect during odontogenic differentiation. TNF-α (10 ng/ml) was added into the medium for 3 days. Western blot analysis indicated that the level of RICK protein was higher after TNF-α treatment (Figure 3; *p < .05).
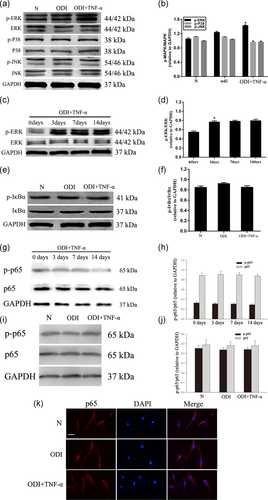
3.3 ERK/p-ERK signaling pathway was activated during the odontogenic differentiation of DPSCs after being treated with TNF-α
DPSCs were cultured with ODI or ODI + TNF-α (10 ng/ml) for 3 days. We found that p-ERK increased significantly in the TNF-α treated group. The JNK and p38 pathways were not activated in any of the groups (Figure 4a,b; *p < .05). To get a better understanding of the ERK pathway in the differentiation of DPSCs, we tested the phosphorylation of ERK at 0, 3, 7, and 14 days. The phosphorylation of ERK increased significantly after the TNF-α treatment from Day 3 (Figure 4c,d; *p < .05). Furthermore, we investigated whether phosphorylation of IκBα (p-IκBα) participated in the process. Western blot analysis showed that the phosphorylation of IκBα did not change significantly in the differentiation of DPSCs (Figure 4e,f; *p < .05) and the expression of p65 and phosphorylation of p65 did not change during the differentiation of DPSCs (Figure 4g–j; *p < .05). Moreover, immunocytochemical staining found that TNF-α treatment did not change the level of p65 in the nucleus (Figure 4k; *p < .05). These results showed that ERK/p-ERK signaling pathway was activated during the odontogenic differentiation of DPSCs, while the NF-κB signaling pathway was not.
3.4 Inhibition of the ERK pathway suppressed the odontogenic differentiation of DPSCs treated with TNF-α
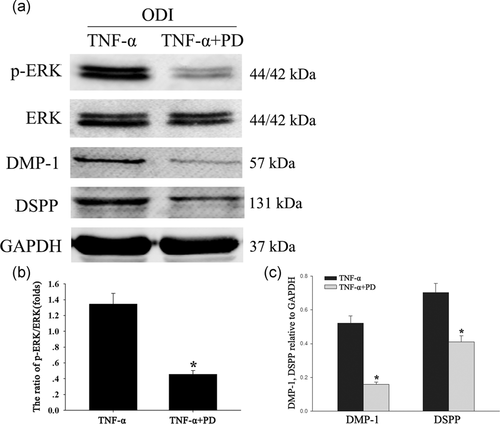
To test the role of the ERK signaling pathway in odontoblastic differentiation of DPSCs, the cells were treated with the ERK signaling pathway inhibitor PD98059. The DPSCs were cultured with ODI + TNF-α for 3 days, and then PD98059 was added to the experimental group. The results showed that, in the presence of PD98059, the phosphorylation of ERK was inhibited and the expression level of DMP-1 and DSPP was reduced (Figure 5a–c; *p < .05).
3.5 Inhibition of RICK reduced the odontogenic differentiation of DPSCs
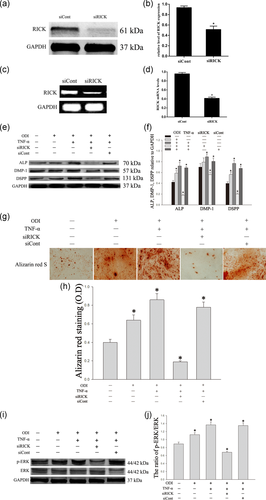
SiRNAs have been used to knock down the expression of RICK. The silencing effect of siRNA was verified by western blot and RT-PCR analyses (Figure 6a–d; *p < .05). All DPSCs were cultured in an odontogenic differentiated medium for 3 days with TNF-α (10 ng/ml). The expression of ALP, DMP-1, and DSPP were downregulated significantly when RICK was inhibited (Figure 6e,f; *p < .05). Alizarin red S staining also indicated that mineralized nodules were reduced significantly when RICK was inhibited (Figure 6g,h). Furthermore, western blot has also shown that the phosphorylation of ERK was significantly lower in the group of siRICK than that in the control group (Figure 6i,j; *p < .05).
4 DISCUSSION
In this study, we demonstrated that 10 ng/ml TNF-α stimulated odontogenic differentiation of DPSCs but did not affect cell proliferation. RICK upregulated the expression of the odontogenic markers, ALP, DMP-1, and DSPP, indicating a positive effect on odontogenic differentiation in TNF-α (10 ng/ml)-treated DPSCs. Furthermore, the ERK signaling was activated in the process of odontogenic differentiation of DPSCs mediated by TNF-α. These findings showed that RICK could promote odontogenic differentiation and mineralization of DPSCs with TNF-α via ERK signaling, not the NF-κB signaling pathway.
TNF-α is an inflammatory cytokine with numerous effects in inflammatory diseases, such as caries, pulpitis, and periodontitis. Detection of TNF-α may be an objective marker to judge the degree of pulpitis (Ding et al., 2019; R. Wang et al., 2020). The elevated TNF-α, which could induce pulp injury during the inflammatory response, has been found in both the pulpitis tissue and the surrounding fluid of patients with caries or irreversible pulpitis (Hirsch et al., 2017; Wu et al., 2018). Furthermore, the upregulated inflammatory cytokines, including TNF-α, were mainly generated in the odontoblast layer (Horst et al., 2011). It has been reported that cultured human odontoblasts from decayed teeth showed 30-fold higher expression of TNF-α compared with that of healthy teeth (Veerayutthwilai et al., 2007), and TNF-α has been regarded as a key mediator, which can promote the dentinogenesis of DPSCs. Our study found that high-level TNF-α (50, 100 ng/ml) treatment had a negative effect on odontogenic differentiation of DPSCs, while low-level TNF-α (1, 10 ng/ml) induced odontogenic differentiation of DPSCs, especially at 10 ng/ml. Previous studies also showed that TNF-α does not affect cell proliferation (P. Li et al., 2017; Xu et al., 2018).
RICK is related to a variety of inflammatory diseases, including inflammatory bowel disease, Blau syndrome, asthma, and arthritis (Talreja et al., 2016; Watanabe et al., 2019; Yue et al., 2019). Previous studies have shown that RICK could lead to intestinal injury induced by muramyl dipeptide (MDP; G. Ma et al., 2015) and also participate in bone destruction caused by osteoclasts (Yamashita et al., 2006). In this study, we found that the expression of RICK increased during odontogenic differentiation of DPSCs after stimulation with TNF-α (10 ng/ml). We therefore speculated that RICK was involved in the above biological process. We found that the knockdown of RICK by siRNA led to a significant decrease in the expression of DMP-1 and DSPP.
In a model of children with congenital heart disease, RICK could activate the downstream pathways, including the NF-κB/p65 and MAPK/p38 pathway, and then initiate the innate immune and inflammatory responses to prevent bacterial infection (Q. Yang et al., 2016). In an experiment of monocytic THP-1 cells induced by MDP, the expression of RICK was promoted after stimulation with TNF-α, and RICK could activate the MAPK signaling pathway subsequently (Chen et al., 2018). In an earlier study, sustained activation of MAPK/ERK signaling was partly dependent on RICK activity in non-small cell lung cancer (S. M. Kim et al., 2016). RICK was also found to be involved in porcine reproductive and respiratory syndrome virus infection in MARC-145 cells. In this experimental system, RICK induced a pro-inflammatory response by NF-кB and MAPK pathways and the secretion of inflammatory cytokines (Jing et al., 2014). Multiple signaling pathways have been shown to contribute to the complexity of odontogenic differentiation induced by TNF-α. RICK is an initial factor in the activation of MAPK and NF-κB signaling pathways, which are downstream of the TNF-α receptor family and the toll-like receptor (TLR)/interleukin-1 (IL-1) receptor family (Hwang et al., 2019; B. Ma et al., 2018). RICK is able to regulate the downstream signal through the above pathways (Jeong et al., 2013; H. S. Kim et al., 2013). ERK1/2 phosphorylation is observed in the differentiation of MSCs to multiple lineages (Yan et al., 2015; Zhang et al., 2015). The ERK signaling pathway is involved in odontogenic differentiation of stem cells originating from the apical papilla (SCAPs) and exfoliated deciduous teeth (SHED; Liu et al., 2019; Mu et al., 2014).
The role of the ERK signaling pathway in the odontogenic differentiation of DPSCs has also been determined in previous studies. The expression of p-ERK increased during TNF-α treatment, while the expression of p-IκBα was without change. Reduced levels of p-ERK are commonly demonstrated with decreasing concentrations of RICK. The expression of DMP-1 and DSPP decreased when the activity of ERK was inhibited. We detected that the odontogenic differentiation of DPSCs treated with TNF-α was significantly increased by the activation of ERK but not NF-κB. How TNF-α regulates the activity of RICK and whether RICK is involved in other biological functions would be studied in the future.
ACKNOWLEDGMENTS
This study was supported by the Health Commission of Jiangsu Province (H2018026), Health Commission of Nantong City (MSZ18085, MSZ19140), and the Science Innovation Foundation of Nantong (JC2018044).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
All procedures performed in the studies involving human participants were approved by the Ethical and Welfare Committee of the Affiliated Hospital of Nantong University (permit number 2016-077).
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.




