YAP nuclear-cytoplasmic translocation is regulated by mechanical signaling, protein modification, and metabolism
Abstract
Nuclear-cytoplasmic transport is necessary for the biological function of nuclear proteins. The mechanism underlying this process is very complex and has been a subject of intense research. Yes-associated protein (YAP), a Hippo signaling pathway effector, localizes to both the cytoplasm and the nucleus and can influence cell proliferation, stem cell status, and tissue homeostasis. Recent studies have focused on the significance of YAP distribution between the nucleus and the cytoplasm in disease, but it remains unclear how this dynamic process is regulated. In this review, we discuss YAP nuclear-cytoplasmic transport under different physiological and pathological conditions in terms of mechanical signaling, protein modification, and metabolism. Understanding the mechanisms underlying nuclear-cytoplasmic YAP transport mechanism under different physiological and pathological conditions may help identify important targets for disease treatment.
Abbreviations
-
- ANKRD1
-
- ankyrin repeat domain 1
-
- ARM
-
- armadillo
-
- CoCl2
-
- cobalt chloride
-
- CRM1
-
- chromosome region maintenance 1
-
- CTGF
-
- connective tissue growth factor
-
- CYR61
-
- cysteine-rich angiogenesis inducer 61
-
- DF
-
- disturbing blood flow
-
- EC
-
- endothelial cells
-
- ECM
-
- cell–extracellular matrix
-
- FAS
-
- fatty acid synthase
-
- FG
-
- Phe-Gly
-
- HFD
-
- high-fat diet
-
- HMGCR
-
- mevalonate-HMG CoA reductase
-
- HUVECs
-
- human umbilical vein endothelial cells
-
- LATS1
-
- large tumor suppressor 1
-
- LF
-
- laminar flow
-
- LINC
-
- Linker of the nucleoskeleton and cytoskeleton
-
- LPA
-
- lysophosphatidic acid
-
- MST1
-
- mammalian STE20-like kinase 1
-
- NE
-
- nuclear envelope
-
- NES
-
- nuclear exceeding signal
-
- NLS
-
- nuclear localization signal
-
- NPC
-
- nuclear pore complex
-
- NTRs
-
- nuclear transporters
-
- NUPs
-
- nucleoporins
-
- O-GlcNAc
-
- O-linked β-N-acetylglucosamine
-
- OGT
-
- O-GlcNAc transferase
-
- PA
-
- phosphatidic acid
-
- S1P
-
- serum-derived sphingosine-1
-
- Ser
-
- serine
-
- SREBP
-
- sterol regulatory element-binding proteins
-
- TF
-
- transcription factors
-
- Thr
-
- threonine
-
- XPO1
-
- exportin 1
-
- YAP
-
- Yes-related protein
Introduction
The Hippo signaling pathway is highly conserved (Hong and Guan, 2012). This pathway consists of a series of kinase cascades including mammalian STE20-like kinases 1 and 2 (orthologs of Drosophila Hippo 1 and 2), which bind to SAV1 and induce the downstream molecules large tumor suppressor 1 and 2 to phosphorylate YAP. The phosphorylated YAP is then able to perform its important cellular roles (Totaro et al., 2018).
YAP is an important effector of the Hippo pathway, and plays important roles in regulating cell proliferation, migration, and differentiation (Feng et al., 2016; Huang et al., 2016). The role of YAP in organ development, tumorigenesis and metastasis, and regenerative medicine has received increasing attention (Zhu et al., 2018; Moya and Halder, 2019; Wu et al., 2019). YAP, which is a transcriptional coactivator, is distributed in both the nucleus and cytoplasm, and its localization affects its function. Interestingly, YAP distribution between the nucleus and cytoplasm is not static (Manning et al., 2018). In Drosophila, Yorkie (the homolog of mammalian YAP) transits rapidly between the cytoplasm and nucleus, rather than being statically located in either compartment.
In this review, we will first discuss the structural and signaling factors that affect YAP nuclear transport and then describe the mechanisms of YAP translocation in terms of mechanical signaling, protein modification, and metabolism. The aim of this review is to demonstrate how YAP nuclear import and export affect its function, and identify potential new targets for treating hepatocellular carcinoma, gastric cancer, atherosclerosis, and angiogenesis (Wang et al., 2017; Li et al., 2019; Niu et al., 2019; Yan et al., 2019).
The structural basis of cell nuclear transport process
The nucleus of a eukaryotic cell is surrounded by two lipid membranes, the inner membrane, and the outer membrane, which together are called the nuclear membrane. The inner and outer nuclear membranes are continuous with those of the endoplasmic reticulum. A fibrous layer underlying the inner nuclear membrane helps maintain nuclear structure, protects the chromatin, and converts mechanical forces from the cytoplasm into nuclear signals (Gruenbaum and Foisner, 2015; De Magistris and Antonin, 2018). A variety of proteins are distributed throughout the nuclear membrane, including laminins A, C, B1, and B2, which constitute the fibrous layer, as well as many transmembrane proteins (NETs) (de Leeuw et al., 2018). The NETs in the outer membrane interact with the cytoskeleton, while the NETs/laminins in the inner membrane interact with chromatin and proteins that regulate gene expression (Korfali et al., 2012).
The nuclear pore complexes (NPCs) create a channel through both the inner and outer nuclear membranes (Raices and D'Angelo, 2012). Due to their massive size, NPCs embedded in the nuclear membrane can be easily observed using an electron microscope (EM). At 2–3nm resolution, the core structure of an NPC can be seen and appears as multiple porous rings with different densities, which are referred to as the cytosolic, internal, and nuclear rings (CR, IR, NR) based on their position within the membrane. On the nuclear side, elongated rod-like extensions connect adjacent rings to form a basket structure. On the cytoplasmic side, eight scaffold extensions of the CR bind to a small number of transmembrane nucleoporins NUPs, which anchor the NPC to the nuclear membrane. This scaffold structure comprises 50 styrene polypropylene fibers. Phenylalanine-glycine (FG)-rich repeats within the NPCs channel control the selective transport of molecules across the nuclear membrane via specific interactions with nuclear transporters (Knockenhauer and Schwartz, 2016; Kim et al., 2018) (Figure 1).
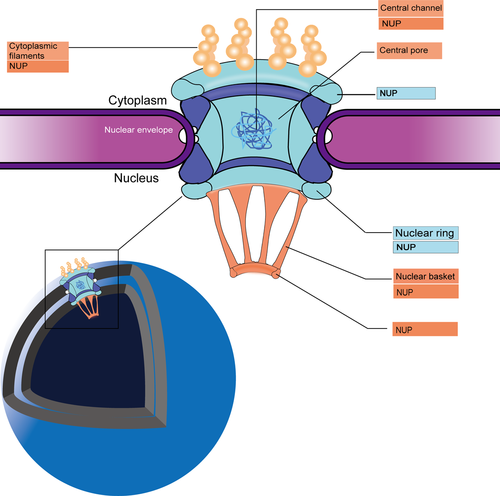
Protein transport into and out of the nucleus requires a nuclear transport receptor and a specific transport signal, for example, a nuclear localization signal (NLS) or a nuclear export signal (NES). NLSs are short amino acid sequences and can be divided into two categories: classical and non-classical sequences. Classical NLSs consist of a cluster of basic amino acids or two basic residues separated by 10–12 neutral residues; and non-classical NLSs lack the basic amino acid segment (Sun et al., 2016). In the NLS-mediated nuclear transport, the NLS peptide first binds to the armadillo domain of the adaptor protein importin α and then dimerizes with the importin β to form a nuclear pore–targeting complex. This ternary complex then docks at the NPC and mediates nuclear import of the cargo molecules via Ran-GTP–dependent active transport. This active transport is triggered by the Ran-GTP–Ran-GDP gradient (Turner et al., 2014). Chromosome region maintenance 1 (CRM1), also known as exportin 1, which is the major nuclear exporter, controls the nuclear export of many proteins and RNA molecules, including all proteins over 40 kDa (Zhang et al., 2014). For a protein to be exported from the nucleus, it must contain a hydrophobic NES, which binds to Cys528 within the active site (hydrophobic trench) of CRM1. The CRM1–cargo protein complex then binds Ran-GTP to form a trimer, which mediates active transport out of the nucleus via diffusion (Turner et al., 2014).
Evaluation of Yorkie kinetics using a Marts mutant showed that the Hippo pathway regulated Yorkie subcellular distribution, primarily by regulating the rate of its nuclear import (Manning et al., 2018). In addition, Yorkie localization changes throughout the cell cycle: in the interphase of cell division, it is primarily located in the cytoplasm, but is enriched in chromatin during mitosis (Manning et al., 2018). The 55 amino acids at the N-terminus of Drosophila Yorkie, especially Arg-15, function as the NLS and mediate its nuclear localization by direct interaction with Importin α1; and its nuclear import can be inhibited by Hippo, an upstream element of the same signaling pathway (Wang et al., 2016b) (Figure 2).There is also a NSE in YAP structure that mediates its export of nuclear. Many sites on NSE can regulate and influence the export process, such as K342 (Fang et al., 2018) (Figure 2).
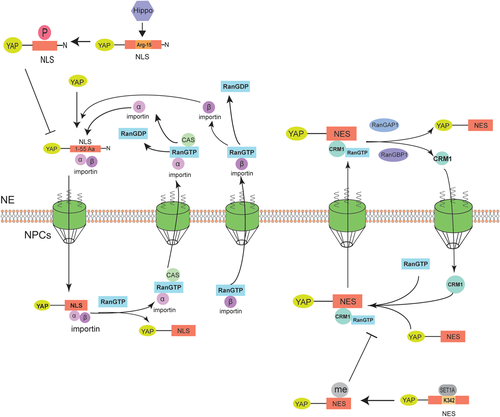
Thus, we summarized other reports on YAP nuclear transport in the following and made our suggestions for taking YAP nuclear transport as a therapeutic target.
Mechanical signals that regulate YAP nuclear import
Cell proliferation and differentiation occur in response to signals from the cellular microenvironment, including physical signals and chemical signals transmitted from other cells or from the extracellular matrix. Mechanical signals, in particular, have recently garnered much attention. Pulling, compressing, and shearing are common mechanical stresses experienced by cells, and cells detect these types of signals through transmembrane structures such as integrins (cell–extracellular matrix focal adhesions), cadherin or adhesive junctions (AJs) (cell–cell adhesion), and tension-activated ion channels. Cytoskeleton remodeling can also transmit these signals. Cytoskeletal components exposed to force, such as actin stress fibers, transmit mechanical signals directly to the NE and nuclear layer through the linker of the nucleoskeleton and cytoskeleton complex that connects the nuclear backbone to the cytoskeleton. The resulting changes in nuclear stiffness and shape can subsequently affect gene expression because of the emerin phosphorylation induced by NE deformation or because of chromatin segregation (Kassianidou et al., 2019).
YAP is one of the most sensitive transcription factors (TFs) activated in response to mechanical stress. Studies have shown that pharmacological factors and altered extracellular matrix stiffness can regulate YAP nuclear localization by altering cell contractility (Thomasy et al., 2013). F-actin is the main component of a variety of cytoskeletal structures, and myosin is the molecular motor of the cytoskeleton. Studies have indicated that, in the absence of cell–cell contact, actin contraction inhibits YAP phosphorylation at Ser (112). Surprisingly, YAP nuclear transport will still occur even if actin does not contract or YAP is not phosphorylated. Changes of actin cytoskeletal integrity seem more critical for YAP nuclear localization, as this stimulus can override YAP regulation by phosphorylation or actin contraction (Das et al., 2016). These data suggest that two different pathways regulate YAP nuclear import in response to mechanical signals transmitted through the cytoskeleton: one that induces YAP phosphorylation in response to actin-myosin contraction and one in which YAP nuclear localization is mediated by cytoskeletal integrity, the latter of which appears to be more important.
YAP and TAZ are homologs, and are the mammalian counterparts of Drosophila Yorkie. TAZ is a transcriptional factor that contains a 14-3-3 binding motif, a single WW domain, and a PDZ binding motif. 14-3-3 is a eukaryotic adaptor protein that binds to other proteins to assist with protein folding, regulate protein localization, and stimulate or inhibit other protein–protein interactions (Stevers et al., 2018). The WW domain mediates protein–protein interactions, which is essential for YAP and TAZ function and can interact with the PPxY motif within many chaperone proteins (such as LATS1, ErbB4, and AMOT) (Webb et al., 2011). The PDZ domain is one of the most common peptide-binding domains and plays an important role in ligand selection, ligand interactions, and association with other protein interaction domains (Manjunath et al., 2018). Recently, the role of YAP/TAZ in many conditions such as atherosclerotic has also been reported (Wang et al., 2016a). Therefore, YAP/TAZ, as a functional unit, has recently come to be considered a promising therapeutic target.
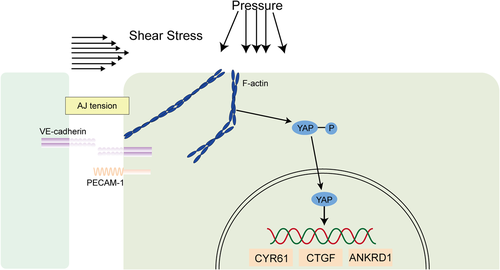
Atherosclerosis typically occurs in the aortic arch and arterial branches, where endothelial cells are exposed to turbulent blood flow. However, it rarely occurs in the thoracic aorta, where stable laminar flow is predominant. Therefore, stable LF and high shear stress protect against developing atherosclerosis (Xu et al., 2016). A growing number of studies have focused on the role of YAP or YAP/TAZ in changes in endothelial cell function triggered by the mechanical force of blood flow. In zebrafish and mouse models of atherosclerosis and an in vitro model of human umbilical vein endothelial cells (HUVECs), YAP/TAZ nuclear localization and the target gene expression are increased in atherosclerotic regions (Xu et al., 2016; Nakajima et al., 2017; Chitragari et al., 2018). Cultured human endothelial cells exposed to turbulent blood flow that promotes atherosclerosis can lead to YAP/TAZ activation and nuclear import, resulting in the upregulation of target proteins such as cysteine-rich angiogenesis inducer 61 (CYR61), connective tissue growth factor (CTGF), and ankyrin repeat domain 1 (ANKRD1) (Wang et al., 2016a). In addition, endothelial cells subjected to normal blood flow maintain vascular stability in response to the lamellar shear stress signal, which is transmitted through filamentous actin and myosin instead of Hippo signaling and induces YAP nuclear import, which promotes its transcriptional activity (Nakajima et al., 2017). Other studies have found that, when HUVECs are exposed to stable laminar flow, the level of phosphorylated YAP is significantly reduced, while the total YAP level remains almost unchanged; conversely, when HUVECs are exposed to a uniform pulsing pressure or turbulent flow, significant reductions in both phosphorylated YAP and total YAP can be observed (Chitragari et al., 2018) (Figure 3). However, the mechanism underlying these phenomena is still unclear.
Metabolic regulation of YAP nuclear transport
In recent years, cell metabolism (such as glucose metabolism, lipid metabolism, and amino acid metabolism) has become an area of intense research in the context of diseases such as cancer, angiogenesis, and atherosclerosis. Recently, glucose, fatty acids, hormones, and other metabolic factors have been found to regulate YAP and TAZ. In addition, YAP and TAZ are involved in metabolic regulation, such as the promotion of glycolysis, lipogenesis and glutamate decomposition, indicating that they are involved in coordinating metabolism, cell growth, and tissue homeostasis (Koo and Guan, 2018). In this section, we will discuss how glucose metabolism and lipid metabolism regulate YAP nuclear import.
Glucose metabolism
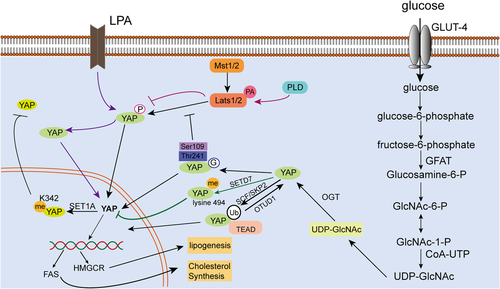
Active glycolysis is a significant biochemical feature of malignant tumor cells. Even under aerobic conditions, malignant cells still exhibit very active glycolysis, a phenotype that is referred to as the Warburg effect (Liberti and Locasale, 2016). Glycolysis in tumor cells is associated with YAP; yap −/− mutant zebrafish exhibit defects in liver progenitor cell potential and liver growth because of impaired glucose transport and nucleotide biosynthesis. Further, transcriptomics and metabolomics analyses revealed that YAP can regulate expression of the glucose transporter glut1, resulting in reduced glucose uptake and nucleotide biosynthesis in embryonic yap−/− mutant zebrafish, as well as decreased glucose tolerance in adult zebrafish. Nucleotide supplementation can partially rescue the yap−/− phenotype (Cox et al., 2018). In vitro and in vivo assessment of neonatal cardiomyocyte repair and regeneration revealed that YAP1 was activated when glycolytic metabolism was enhanced, whereas inhibition of glycolysis suppressed YAP activation (Wang et al., 2018). O-GlcNAcylation is a specific type of post-translational modification catalyzed by O-GlcNAc transferase (OGT), resulting in the transfer of O-linked β-N-acetylglucosamine (O-GlcNAc) to the hydroxyl group of serine (Ser) or threonine (Thr) residues in the target protein. Since both O-GlcNAcylation and phosphorylation target Ser and/or Thr side chains, they can compete for the same Ser/Thr site within the substrate, or can selectively target different Ser/Thr residues (Zhang et al., 2017). O-GlcNAcylation modification of YAP plays an important role in the development of liver tumors whose growth is stimulated by high glucose. Mechanically, O-GlcNAcylation can enhance YAP expression, stability, and function. The Thr241 residue of YAP is modified by O-GlcNAc, and mutating this site reduces YAP O-GlcNAcation, stability, and tumorigenic ability, but increases its phosphorylation (Zhang et al., 2017). In addition, under conditions of high glucose metabolism, YAP can be O-GlcNAcylated at Ser109, which disrupts its ability to bind to the upstream kinase LAST1, prevents YAP from being phosphorylated, and activates its transcriptional activity (Peng et al., 2017) (Figure 4).
Lipid metabolism
Animal experiments have shown that the offspring of mothers fed with a high-fat diet (HFD) exhibit little YAP activity. In rat embryos and pups exposed to an HFD before birth, there was a significant reduction in YAP messenger RNA and protein in the paraventricular nucleus of the hypothalamus (Poon et al., 2013). Phosphatidic acid (PA) is a lipid second messenger that binds directly to intracellular proteins and regulates intracellular signaling pathways, thereby affecting cell adhesion, cell migration, membrane remodeling, and cell polarization. PA-associated lipid signaling is a key regulator of the Hippo signaling pathway. Supplementation with PA can activate YAP by activating Hippo signaling (Han et al., 2018). Furthermore, PA can directly interact with the Hippo signaling pathway components LATS and NF2 to disrupt LATS-MOB1 complex formation and NF2-mediated LATS membrane translocation and activation, respectively, resulting in inhibition of LATS kinase activity and upregulation of the nuclear activity of the transcriptional regulator YAP (Han et al., 2018). Lysophosphatidic acid (LPA), which is the smallest and simplest phospholipid, as well as a key precursor in the early stages of eukaryotic phospholipid biosynthesis, has been found to stimulate YAP/TAZ transcriptional activity in HTM cells by regulating cell contraction and increasing CTGF expression, which, in turn, leads to an increase in ECM production (Park et al., 2016; Ho et al., 2018). Furthermore, YAP/TAZ activity can be regulated by serum-derived sphingosine-1-phosphate (S1P), LPA, GPCR signals, and the actin-myosin cytoskeleton. S1P and LPA can induce YAP dephosphorylation and nuclear localization, S1P2 receptor activation, Rho GTPase activation, and F-actin polymerization (Miller et al., 2012).
Hypoxia is widely recognized as a common feature of a variety of solid tumors and is closely related to tumor cell proliferation, differentiation, apoptosis, and phenotypic determination, as well as tumor metabolism, angiogenesis and resistance to treatment, and patient prognosis. Hypoxia can induce YAP nuclear translocation and increase the resistance of hepatocellular carcinoma cells to SN38, an anti-tumor drug (Dai et al., 2016). However, hypoxia-induced YAP activation is not mediated by HIF-1α, as YAP phosphorylation and expression levels are not affected by cobalt chloride (CoCl2) concentrations. Rather, YAP activation under hypoxic conditions is regulated by the mevalonate-HMG CoA reductase (HMGCR) pathway (Dai et al., 2016). The HMGCR-mediated cholesterol synthesis pathway can also be regulated by YAP. In hepatocyte nuclei, YAP can directly interact with the promoters of the genes encoding sterol regulatory element-binding proteins (SREBP-1c and SREBP-2)––components of the fatty acid synthase (FAS) pathway––and HMGCR to stimulate their transcription and promote hepatocyte lipogenesis and cholesterol synthesis (Shu et al., 2019). In addition, LATS2, a tumor suppressor that is upstream of YAP, can also inhibit liver cholesterol accumulation by inhibiting SREBP (Aylon et al., 2016). TAZ/YAP activity is positively correlated with transaminase expression in patients with breast cancer, suggesting that transaminase is a potential target for treatment in patients with TAZ/YAP-driven breast cancer (Yang et al., 2018) (Figure 4).
Effect of YAP modification on YAP nuclear transport
In the previous section, we discussed the mechanism by which proteins are transported from the cytoplasm to the nucleus. YAP nuclear import and export occur because of its structure, and modification of the YAP structure prevents these processes. Activation of the Hippo signaling pathway kinase cascade results in YAP phosphorylation by LATS and prevents YAP from entering the nucleus (Sun and Irvine, 2016). However, in addition to phosphorylation, methylation, acetylation, and ubiquitination also prevent the transfer of YAP into the nucleus. The lysine methyltransferase Set7 (Setd7), which contains a SET domain, induces YAP retention in the cytoplasm by monomethylating the YAP lysine 494 residue (Oudhoff et al., 2013). SET1A-mediated K342 monomethylation can also block YAP nuclear export, thereby regulating YAP activation and promoting tumorigenesis (Fang et al., 2018). SETD7 is part of a complex containing YAP, AXIN1, and β-catenin, and SETD7-dependent methylation of YAP promotes Wnt induction and nuclear accumulation of β-catenin, which consequently controls intestinal regeneration and tumorigenesis by regulating Hippo/YAP signaling (Oudhoff et al., 2016). In addition, YAP phosphorylation mediated by the Hippo pathway is significantly reduced by treating cells with the S(N)2 alkylating agent methyl methanesulfonate, although these cells exhibit YAP acetylation in the nucleus. This YAP acetylation modifies a specific and highly conserved C-terminal lysine residue and is mediated by nuclear acetyltransferase CREB-binding protein (CBP) and p300 (Hata et al., 2012). Ubiquitination is another important post-translational modification. The ubiquitin-proteasome system mediates 80–85% of protein degradation in eukaryotes and can regulate a variety of cellular activities including cell cycle, apoptosis, transcriptional regulation, DNA damage repair, and the immune response. It has recently been found that YAP is non-proteolytically ubiquitinated by the SCFSKP2 E3 ligase complex and polyubiquitinated by K63, and that these modifications can be reversed by the deubiquitinating enzyme OTUD1. Non-proteolytic ubiquitination of YAP enhances its interaction with its nuclear-binding partner TEAD, thereby inducing YAP nuclear localization, transcriptional activity, and cell growth promotion (Yao et al., 2018a, 2018b) (Figure 4).
Conclusion
In this review, we discussed how mechanical signaling, protein modification, and metabolism influence YAP translocation within the cell. Based on our current understanding, YAP localization is determined not only by Hippo signaling pathway–mediated phosphorylation of YAP by LATS, which results in YAP retention in the cytoplasm, but also by many other signals or molecules that also regulate YAP expression and function. Further exploration of these factors will be meaningful for studying the pathogenesis of many diseases and identifying potential therapeutic targets.
Funding
This study was supported by grants from the National Nature Science Foundation of China (Grants 81600735, 81873680, 81970817, and 81600703).
Conflict of interest
The authors declare that there are no conflict of interest.
Availability of data and materials
Any information used and/or analyzed during this study is available from the corresponding author on reasonable request.




