Fluid shear stress induces Runx-2 expression via upregulation of PIEZO1 in MC3T3-E1 cells
Jidong Song, Liying Liu, Wei Wang, and Xiaoqian Dang contributed equally to this work.
Abstract
Mechanically induced biological responses in bone cells involve a complex biophysical process. Although various mechanosensors have been identified, the precise mechanotransduction pathway remains poorly understood. PIEZO1 is a newly discovered mechanically activated ion channel in bone cells. This study aimed to explore the involvement of PIEZO1 in mechanical loading (fluid shear stress)-induced signaling cascades that control osteogenesis. The results showed that fluid shear stress increased PIEZO1 expression in MC3T3-E1 cells. The fluid shear stress elicited the key osteoblastic gene Runx-2 expression; however, PIEZO1 silencing using small interference RNA blocked these effects. The AKT/GSK-3β/β-catenin pathway was activated in this process. PIEZO1 silencing impaired mechanically induced activation of the AKT/GSK-3β/β-catenin pathway. Therefore, the results demonstrated that MC3T3-E1 osteoblasts required PIEZO1 to adapt to the external mechanical fluid shear stress, thereby inducing osteoblastic Runx-2 gene expression, partly through the AKT/GSK-3β/β-catenin pathway.
Abbreviations
-
- CCK-8
-
- cell counting kit-8
-
- FSS
-
- fluid shear stress
-
- siRNA
-
- small interference RNA
Introduction
Bone is a dynamic tissue continuously remodeling through the balance of osteoblast-mediated formation and osteoclast-mediated resorption in response to the mechanical forces that are experienced. Bone remodeling is an important process for preserving structural integrity, maintaining metabolic homeostasis, and regenerating damaged bone, which is the basis for bone-tissue engineering and orthopedic treatments (Galea et al., 2017). Long-term bedridden patients and astronauts who experience reduced mechanical loading have shown significant bone loss and mineral changes, whereas targeted load application can promote bone formation and reverse the decreased osteoblastic activity in many bone diseases. For example, resistance exercise has been shown to increase total body, lumbar spine and femur neck bone mineral density in patients with glucocorticoid-induced osteoporosis (Braith et al., 2003, 2007). Therefore, understanding the mechanosensitive mechanisms of bone could contribute greatly to the treatments of many skeletal disorders.
Fluid flow caused by compressive loading of bone exerts fluid shear stress (FSS) on the surface of the porous spaces of bone. Mechanical regulation of bone involves complex processes, including the sense of mechanical stimulations applied, their conversion into biochemical cascades, and ultimately adaptive responses of bone cells (Dalagiorgou et al., 2013). Several mechanotransducers have been identified such as integrins, primary cilia, and stretch-activated Ca2+ channels. However, how mechanosensitive molecules sense mechanical signals and transform into biochemical signals remains unclear.
PIEZO1 has been identified as a new Ca2+-permeable and directly mechanically activated ion channel (Coste et al., 2010). PIEZO1 functions as the key mechanotransducer in red blood cells and bladder urothelium, and its mutation has been related to human anemia and xerocytosis (Zarychanski et al., 2012). In skeletal tissue, recent studies have shown that PIEZO-mediated mechanotransduction pathway plays an important role in the mechanical load-mediated chondrocyte death. GsMTx4, the PIEZO1 channel blocker, significantly decreased the “zone of death” surrounding the mechanically induced stab injury of cartilage (Lee et al., 2014). PIEZO1 can also regulate the cell fate determination of mesenchymal stem cells in response to hydrostatic pressure (Sugimoto, 2017). Knockout of PIEZO1 in osteoblasts and osteocytes blunts the skeletal response to mechanical loads and resulted in the impair of bone structure and strength (Li et al., 2019; Sun et al., 2019). And PIEZO1 agonist could increase bone mass in adult mice, simulating the effects of mechanical loading (Li et al., 2019). Also, Miyazaki et al. (2019) found that PIEZO1 functions as a mechanotransducter that connects hydrostatic pressure signal to the intracellular signals during odontoblast differentiation through coordination of WNT signaling and ciliogenesis. Therefore, PIEZO1 may play a vital role in the mechanotransduction process regulating bone homeostasis in response to mechanical loading.
This study was performed to determine whether mechanical stimulation in the form of FSS-induced Runx-2 expression through PIEZO1 and, if so, to investigate the molecular signaling mechanisms is responsible for this effect.
Materials and methods
Reagents
Fetal bovine serum (FBS) were purchased from Biological Industries (Kibbutz Beit Haemek, Israel). Antibody against PIEZO1 (NBP1–78537) was purchased from Novus. Antibodies against Akt (10176-2-AP), phospho-Akt (Ser-473) (66444-1-Ig), GSK-3β (22104-1-AP), Erk1/2 (16443-1-AP), and β-catenin (17565-1-AP) were purchased from Proteintech. Antibodies against phospho-GSK-3β (Ser-9) (MA5–14873), phospho-Erk1/2 (44–680G), and β-actin (MA5–11869) were purchased from Thermo Fisher Scientific (MA, USA). Antibodies against Runx-2 (ab23981) were purchased from Abcam. The secondary antibodies used for western blot (goat anti-mouse W10808 and goat anti-rabbit W10809) and immunofluorescence (Alexa Fluor 594 goat anti-rabbit A11012) were purchased from Invitrogen (MA, USA).
Cell culture and mechanical loading
The mouse osteoblast cell line, MC3T3-E1, was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in α-minimum essential medium (α-MEM) supplemented with 10% FBS, 1% glutamax (Sigma-Aldrich, MO, USA) and 1% penicillin/streptomycin in a humidified incubator (Thermo Fisher Scientific) with 5% CO2 at 37°C. The medium was refreshed every other day. Before experiments, 1× 105/mL cells were plated on culture dishes, grown to 70–80% confluence, and serum-deprived for 24 h.
For mechanical loading, the cells were exposed to FSS by placing T25 flasks or six-well culture dishes on a horizontal shaking apparatus fixed inside a culture incubator and shaken at 100–120 rpm (Kido et al., 2009). The shear stress force was estimated to be slightly less than that produced at 200 rpm with a cone viscometer, which was approximately 2 Pa at the edge (Sakai et al., 1999), and thus, theoretically stayed within the physiological range (Weinbaum et al., 1994).
Western blot
After FSS application, the cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in RIPA supplemented with protease and phosphatase inhibitors (Roche, Basel, Switzerland). Cells extracts were centrifuged at 12,000 rpm for 10 min at 4°C. The concentrations of protein samples were measured according to the absorbance at 280 nm using the ND-1000 spectrophotometer (NanoDrop, DE, USA). The cellular protein (20 µg) was loaded and electrophoresed on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (8–12% gels). Then, the proteins in the gel were transferred to Immobilon-P polyvinylidene difluoride (Millipore, Merck, Germany) for immunoblot analysis and blocked with 5% bovine serum albumin (BSA) for 2 h at room temperature. After that, the membranes were incubated with primary antibodies overnight at 4°C. They were further incubated with goat anti-mouse(or anti-rabbit) IgG H&L (HRP) secondary antibodies (W10808, W10809; Invitrogen) at room temperature for 1 h. Finally, the immunoreactive bands were visualized using the enhanced chemiluminescence (ECL) kit.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNAs were isolated using TRIzol reagent (GenStar, China) according to the manufacturer's protocol and treated with RNase-free ddH2O. The concentration and purity of RNA samples were determined by the absorbance of RNA at 230, 260, and 280 nm. Then, 1.5 μg total RNA was reverse-transcribed using the StarScript II First-strand cDNA Synthesis Mix (GenStar). Relative transcript levels were measured by quantitative PCR in 20-μL reaction volume using a CFX Connect Real-Time PCR Detection System (Bio-Rad, CA, USA), according to the recommended protocol for 2× RealStar Green Power Mixture (GenStar). The expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as an internal control. The primers for PIEZO1 were as follows: forward primer, 5′-TCATCATCCTTAACCACATGGTG-3′; reverse primer, 5′-TGAAGACGATAGCTGTCATCCA-3′. The primers for COX-2 were as follows: forward primer, 5′-TTCAACACACTCTATCACTGGC-3′; reverse primer, 5′-AGAAGCGTTTGCGGTACTCAT-3′. The primers for Runx-2 were as follows: forward primer, 5′-CCAGATGGGACTGTGGTTACC-3′; reverse primer, 5′-ACTTGGTGCAGAGTTCAGGG-3′. The primers for GAPDH were as follows: forward primer, 5′-GGAGCGAGACCCCACTAACATC-3′; reverse primer, 5′-CTCGTGGTTCACACCCATCAC-3′. The primers for Axin2 were as follows: forward primer, 5′-TGACTCTCCTTCCAGATCCCA-3′; reverse primer, 5′-TGCCCACACTAGGCTGACA-3′. The results were quantified using the 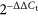 method.
method.
Immunofluorescence
The cells were fixed for 15 min in 4% paraformaldehyde. Following three washes with PBS, they were blocked with 5% BSA in PBS at room temperature for 1 h. They were then incubated with primary antibodies at a 1:100 dilution overnight at 4°C. After three washes with PBS, they were incubated with the appropriate fluorescent-labeled secondary antibodies for 1 h in darkness. Finally, the cells were stained with 0.2 μg/mL 4′,6-diamidino-2-phenylindole (DAPI) in PBS for 5 min and then washed three times with PBS. The slides were examined using an Olympus IX71 inverted fluorescence microscope (Olympus, Tokyo, Japan).
Cell counting kit-8 (CCK-8) assay
For detecting cell proliferation, the CCK-8 assay was used (Zhang et al., 2017). After exposure to FSS, with or without PIEZO1 small interference RNA (siRNA) pre-incubation, MC3T3-E1 cells were collected from the T25 flasks and re-seeded into 96-well plates (20,000 cells/well). After the cells were incubated with the medium containing 10% serum for 24 h, they were loaded with 10 μL CCK-8 solution (C0037; Beyotime Biotechnology, Beijing) and incubated in 5% CO2 at 37°C for 1 h. Optimal density values were measured at 450 nm using an ELx800UV reader (BioTek Instruments, VT, USA).
SiRNA transfection
Three siRNAs targeting PIEZO1 (siRNA1—sense: 5′-CGGACAGCUACAUCAAGUA-3′, antisense: 5′-UACUUGAUGUAGCUGUCCG-3′; siRNA2—sense: 5′-GGGGCAGAAGAAGAAGAAA-3′, antisense: 5′-UUUCUUCUUCUUCUGCCCC-3′; siRNA3—sense: 5′-CGGCACAGCCGACAUUACA-3′, antisense: 5′-UGUAAUGUCGGCUGUGCCG-3′) and a scrambled siRNA (sense: 5′-UUCUCCGAACGUGUCACGUTT-3′, antisense: 5′-ACGUGACACGUUCGGAGAATT-3′) were synthesized by GenePharma Biotechnology (Shanghai, China). MC3T3-E1 cells were transfected with siRNA (50 nM) using a jetPRIME transfection reagent (Polyplus-transfection, NY, USA) following the manufacturer's protocol. The siRNA silencing efficiency was determined 48 h post-transfection by qRT-PCR and western blot for further experiments.
Inhibition of Akt phosphorylation
MK-2206 (M1837; Abmole Bioscience) is a selective inhibitor of Akt phosphorylation (Yang et al., 2015). It was used in a concentration of 5 μmol/L to inhibit the phosphorylation of Akt (Lauzon et al., 2016). MK-2206 was added 2 h before the application of FSS. After 1 h of FSS, western blot was performed to examine the expression of signal molecules.
Statistical analysis
All data were expressed as the mean ± standard deviation and analyzed using SPSS V20.0. All experiments were repeated three times. Data were statistically analyzed using the one-way analysis of variance. A P-value <0.05 indicated a statistically significant difference.
Results
Detection of PIEZO1 expression in MC3T3-E1 cells
First, the expression of PIEZO1 in human and mouse osteoblasts was analyzed using RNA-seq data downloaded from GSE18043 (Hamidouche et al., 2009) and GSE10246 (Lattin et al., 2008). As shown in Figure 1A, PIEZO1 was highly expressed in both human and mouse osteoblasts. Then, the mouse osteoblast cell line MC3T3-E1 was used to detect PIEZO1 expression. Immunofluorescence and inverted fluorescence microscope were used, demonstrating that confluent MC3T3-E1 cells expressed PIEZO1 proteins (Figure 1B). Further, the expression of PIEZO1 protein in MC3T3-E1 cells was validated using western blot (Figure 1C).
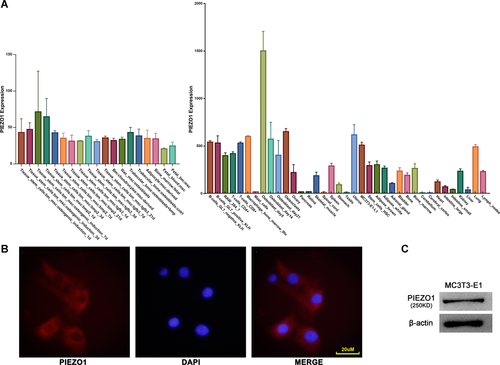
Expression levels of PIEZO1 during 1 h of FSS in MC3T3-E1 cells
To investigate whether the expression of PIEZO1 in MC3T3-E1 osteoblasts was affected by FSS at both messenger RNA (mRNA) and/or protein levels, confluent MC3T3-E1 cell cultures grown on T25 flasks were incubated overnight with a starvation medium and then subjected to the horizontal shaking application for various periods of time.
As depicted in Figure 2, the levels of PIEZO1 protein expression increased during 1 h of FSS with a maximal increase of 2.6-fold after 30 min compared with the static control group and this trend was confirmed by immunofluorescence. However, no changes in the level of PIEZO1 mRNA expression were seen during 1 h of FSS. To verify the response of MC3T3-E1 cells to FSS, the COX-2 gene expression, one of the first genes upregulated in response to FSS (Celil et al., 2010) and known to be rapidly released in response to mechanical loading, was monitored using qRT-PCR. The analysis revealed that 1 h of mechanical stimulation using the horizontal shaking platform induced a 4-fold upregulation of COX-2 gene expression in MC3T3-E1 osteoblasts. This demonstrated that the said system was sufficient to elicit a mechanically induced response in this cell line. Thus, 1 h of FSS applied using the horizontal shaking platform was used for the following experiments.
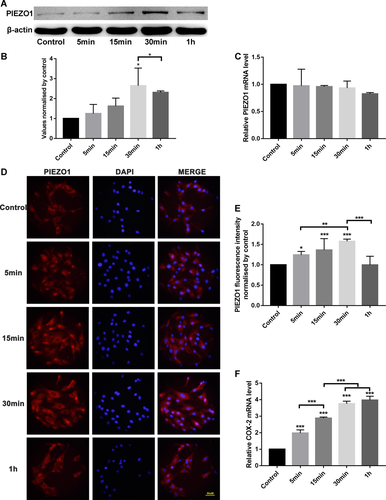
FSS elicited Runx-2 mRNA expression in MC3T3-E1 osteoblasts
To analyze the function of PIEZO1 in MC3T3-E1 cell proliferation, small interference RNA was used to silence the PIEZO1 protein and the proliferation after FSS, with or without PIEZO1 siRNA, was examined. Compared with negative control (NC) siRNA cells, the expression of PIEZO1 mRNA and protein in PIEZO1 siRNA1 cells were declined by 80 and 57%, respectively. Compared with PIEZO1 siRNA2 and siRNA3, the interference efficiency of siRNA1 was the highest. Thus, transfected PIEZO1 siRNA1 cells were used for further experiments (Figure 3A–C).
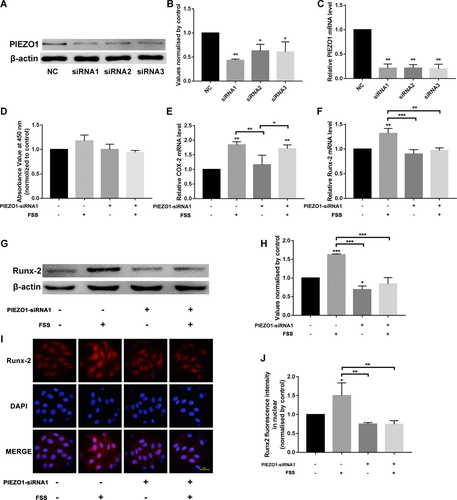
After exposure to FSS, with or without PIEZO1 siRNA1 pre-incubation, the cells were incubated for 24 h and then cell proliferation was detected. The results showed 1 h of FSS-promoted cell proliferation, but with no significant differences compared to the static control group. In contrast, when cells were pre-incubated with PIEZO1 siRNA, the FSS-induced proliferation increase was not seen (Figure 3D). MC3T3-E1 osteoblasts, with or without PIEZO1-siRNA1, were subjected to 1 h of FSS. As depicted in Figure 3E, 1 h of FSS induced the upregulation of COX-2 gene expression in MC3T3-E1 osteoblasts compared with the static control group. However, silenced or unsilenced PIEZO1 had no effect on the FSS-induced COX-2 gene expression, indicating that the silencing of PIEZO1 had no effect on the mechanical stimulation of osteoblasts. Runx-2 is a nuclear protein which is essential for osteoblastic differentiation and bone formation. 1 h of FSS induced a 1.8-fold increase in Runx-2 gene expression in MC3T3-E1 osteoblasts compared with the static control group. The FSS-induced Runx-2 gene expression was eliminated after silencing of PIEZO1 (Figure 3F), indicating that PIEZO1 played an important role in the FSS-induced Runx-2 gene expression. This result was also confirmed by western blot and immunofluorescence (Figure 3G–J).
FSS activated the AKT/GSK-3β/β-catenin signaling pathway via PIEZO1
In this study, western blot analyses showed that 1 h of FSS increased the phosphorylation of Akt (Ser-473). Since the canonical Wnt/β-catenin signaling pathway plays an important role in the modulation of osteoblast osteogenesis, the present study investigated the involvement of the pathway in the FSS-induced Runx-2 expression. Western blot analyses showed that 1 h of FSS increased the phosphorylation of GSK-3β (Ser-9), which inhibited the activity of GSK-3β. However, the silencing of PIEZO1 blocked the FSS-induced upregulation of phosphor-Akt and phosphor-GSK-3β in PIEZO1 siRNA osteoblasts (Figure 4A–C).
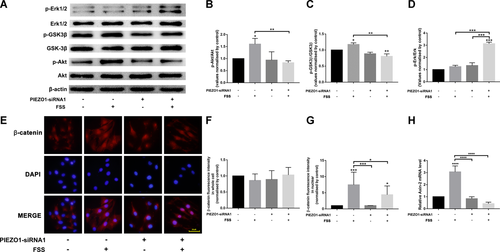
β-catenin is a crucial transcriptional factor in Wnt signaling. It translocates to the nuclear to activate downstream target genes. Immunofluorescence results revealed a weak nuclear fluorescence of β-catenin in the static control group. However, after 1 h of FSS, the fluorescence intensity of nuclear β-catenin significantly increased. This suggested that the mechanical stimulation induced nuclear translocation of β-catenin. However, the silencing of PIEZO1 weakened the FSS-induced nuclear accumulation of β-catenin in PIEZO1 siRNA osteoblasts (Figure 4E–G). We also analyzed the expression of Axin2 mRNA, a direct target of Wnt pathway serving as a negative regulator of this pathway. As shown in Figure 4H, 1 h of FSS induced a threefold increase in Axin2 gene expression in MC3T3-E1 osteoblasts compared with the static control group, while the FSS-induced Axin2 gene expression was eliminated after silencing of PIEZO1.
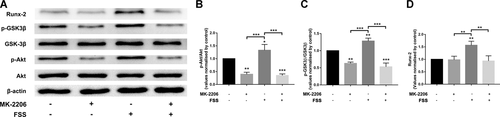
To investigate whether the Akt/GSK-3β pathway is required for the FSS-induced Runx-2 expression, we inhibited Akt phosphorylation by using MK-2206. A quantity of 5 μmol/L MK-2206 was added 2 h before the application of 1 h of FSS. As shown in Figure 5, FSS-induced Akt phosphorylation and subsequent GSK-3β phosphorylation along with upregulated Runx-2 expression were inhibited by the Akt inhibitor MK-2206. This suggested that Akt/GSK-3β pathway is required for the FSS-induced Runx-2 upregulation.
Erk phosphorylation has been shown to be associated with Runx-2 expression and its activation by FSS was biphasic. In our study, interestingly, we found different response of Erk phosphorylation with that of Akt phosphorylation after 1 h of FSS. 1 h of FSS did not increase the phosphorylation of Erk compared with the static control group. However, the upregulation of phosphor-Erk was induced by FSS in PIEZO1 siRNA osteoblasts (Figures 4A and 4D).
Discussion
The present study explored the effects of the mechanosensory molecule, PIEZO1, on Runx-2 gene expression of FSS-induced osteoblastic MC3T3-E1 cells. The study confirmed the presence of PIEZO1 in MC3T3-E1 osteoblasts. The findings supported the promoting role of PIEZO1 on FSS-induced Runx-2 gene expression in osteoblastic cells. This effect was mediated, at least in part, through the activation of Akt/GSK-3β/β-catenin pathway.
PIEZO1 proteins were identified in 2010 as the pore-forming subunits of excitatory mechanosensitive ion channels (Coste et al., 2010). As far as skeletal organs are concerned, the PIEZO-mediated mechanotransduction pathway is important in the mechanical load-mediated cartilage injury (Lee et al., 2014) and cell fate determination of mesenchymal stem cells. The present study showed that PIEZO1 was expressed on the surface of MC3T3-E1 cells; this finding was confirmed by western blot and agreed with the findings of Sugimoto (2017). The results also showed that mechanical stimulation could induce PIEZO1 expression. This agreed with the findings of the studies on chondrocytes and bone mesenchymal stem cells (Sugimoto, 2017; Jones et al., 2018).Therefore, PIEZO1 may play a vital role in the mechanotransduction process regulating bone homeostasis in response to mechanical loading.
Previous studies on mechanosensitive molecules demonstrated a molecular link between mechanical stimulation and bone-specific transcription factor function such as Runx-2 gene expression (Xiao et al., 2018). Runx-2, the key nuclear protein regulating of osteoblastic differentiation and rate of bone formation, has been suggested to be involved in the bone's adaptive response to mechanical stimulation (Costessi et al., 2005). A selective knockout of Runx-2 could block mechanically induced bone formation in vivo (Salingcarnboriboon et al., 2006). In vitro, a number of signaling pathways have been demonstrated to be involved in the induction of Runx-2 by mechanical stimulation in osteoblasts, such as ERK-dependent pathway (Ziros et al., 2002; Jiang et al., 2015). However, the molecular mechanisms underlying the mechanical induction of Runx-2 still remain unclear. Especially, the “primum movens” responsible for the induction of the signaling cascade leading to the expression of Runx-2 on mechanical loading has not yet been elucidated. Indeed, the present study revealed the modulation of Runx-2 gene expression by PIEZO1-mediated mechanical stimulation. The mRNA, western blot, and immunofluorescence analyses of FSS-induced osteoblastic cells in the presence or absence of PIEZO1-siRNA unveiled a link between PIEZO1 and Runx-2 gene expression levels. We used COX-2 as the marker for mechanical reactions (Jiang et al., 2015). However, the silencing of PIEZO1 had no effect on the FSS-induced COX-2 gene expression (Figure 3E). This implied that PIEZO1 may not be the only mechanosensitive molecule in osteoblasts because COX-2 gene expression can be induced through other mechanosensors and signaling pathways (Wadhwa et al., 2002; Hoey et al., 2011).
Mechanical stimulation sensed by cells must be translated into biochemical changes in signaling events such as phosphorylation, transcription factor translocation, or alterations of gene expression. Consequently, the present study focused on the Akt/β-catenin signaling pathway, an important signaling pathway implicated in the control of cell differentiation and cellular adaption (Duan and Bonewald, 2016), whose regulatory effect on Runx-2 expression and activity has been demonstrated by several studies (Hamidouche et al., 2008; Haxaire et al., 2016; Vega et al., 2017). β-catenin is the central, critical molecule in the canonical Wnt/β-catenin pathway, which plays a key role in osteoblast differentiation and proliferation (Duan and Bonewald, 2016). GSK-3β is phosphorylated by p-Akt, and the degradation of β-catenin is inhibited. β-catenin accumulates in the cytoplasm and then translocates to the nuclei where it activates genes that regulate osteoblastic homeostasis such as Runx-2. Previous studies have shown that the Wnt/β-catenin pathway regulates the expression and activity of Runx-2 (Hamidouche et al., 2008; Cai et al., 2016). Intracellular Ca2+ is related to the phosphorylation of Akt. In addition, PIEZO1 has been reported to serve as the Ca2+-permeable channel in response to mechanical stimuli. Axin2 is an important component of the Wnt pathway, which functions as a negative regulator of the pathway by promoting the phosphorylation and consequent degradation of β-catenin (Kikuchi, 1999). It has been shown that Axin2 could be induced by activation of the Wnt pathway (Jho et al., 2002). In our study, the FSS-induced Axin2 upregulation was blocked by PIEZO1 deletion, which was paralleled with FSS-induced activation of the Wnt pathway. This provided another proof that Wnt signaling is affected by PIEZO1 deletion. Akt inhibitor inhibited the FSS-induced Wnt pathway activation as well as Runx-2 expression. This suggested that Wnt pathway is required for the FSS-induced Runx-2 upregulation.
Different from the response of Akt phosphorylation to FSS, FSS did not increase the Erk phosphorylation in our study. Erk activation by mechanical stimuli has been shown to be biphasic, while Akt activation is monophasic (Liu et al., 2006; Fukada et al., 2017). Fukada et al. (2017) found that FSS induced transient early Erk activation and persistent late Erk activation after an intermittent phase. The non-response of Erk phosphorylation in our study agreed with Fukada's finding and this may be because that the Erk activation was in the intermittent phase under our experimental conditions. However, in our study, the upregulation of phosphor-Erk was induced by FSS in PIEZO1 siRNA MC3T3-E1 cells. This may be because that PIEZO1 knockdown makes osteoblasts insensitive to mechanical stimuli and makes osteoblasts no longer in the intermittent phase. Erk1/2 and Akt play different roles in cells in response to mechanical stimuli. It has been shown that Akt phosphorylation functions as a survival signal (Datta et al., 1997), while Erk activated by moderate stretch and functions as a differential signal (Matsui et al., 2014). Our results imply that FSS-induced Runx-2 expression may be independent of Erk1/2 signaling. The different mechanotransductal effect between Erk1/2 and Akt agreed with Song's and Watabe's study (Watabe et al., 2011; Song et al., 2016). The specific mechanism needs further research.
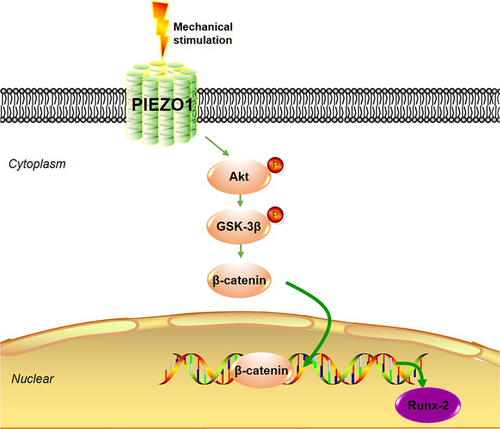
In conclusion, the results of this study can be integrated into a schematic model that links PIEZO1 to the induction of Runx-2 gene expression via activation of the Akt/GSK-3β/β-catenin signaling pathway under mechanical stimulation (Figure 6). FSS induces Runx-2 expression in MC3T3-E1 osteoblastic cells via PIEZO1. This effect is mediated by the Akt/GSK-3β/β-catenin pathway through PIEZO1. The present study uncovered an important role of PIEZO1 as a key molecule that could sense mechanical stimulation in the form of FSS and regulate signaling pathways, leading to the modulation of osteoblastic gene expression.
Acknowledgments and funding
We would like to thank The Center Laboratory for Biomedical Research of Xi'an Jiaotong University for providing experimental apparatuses. We also thank International Science Editing for editing this manuscript. This study was funded by the Key Research and Development Program of Shaanxi (Program No. 2019SF-070) and the Fundamental Research Funds for the Central Universities (Grant No. xzy022019043).
Conflict of interest
The authors declare that they have no conflict of interest.




