Indospicine combined with arginine deprivation triggers cancer cell death via caspase-dependent apoptosis
Galyna Y. Shuvayeva and Yaroslav P. Bobak contributed equally to this work.
Abstract
Arginine-deprivation therapy is a rapidly developing metabolic anticancer approach. To overcome the resistance of some cancer cells to this monotherapy, rationally designed combination modalities are needed. In this report, we evaluated for the first time indospicine, an arginine analogue of Indigofera plant genus origin, as potential enhancer compound for the metabolic therapy that utilizes recombinant human arginase I. We demonstrate that indospicine at low micromolar concentrations is selectively toxic for human colorectal cancer cells only in the absence of arginine. In arginine-deprived cancer cells indospicine deregulates some prosurvival pathways (PI3K-Akt and MAPK) and activates mammalian target of rapamycin, exacerbates endoplasmic reticulum stress and triggers caspase-dependent apoptosis, which is reversed by the exposure to translation inhibitors. Simultaneously, indospicine is not degraded by recombinant human arginase I and does not inhibit this arginine-degrading enzyme at its effective dose. The obtained results emphasize the potential of arginine structural analogues as efficient components for combinatorial metabolic targeting of malignant cells.
Abbreviations
-
- AFM
-
- arginine-free medium
-
- Arg
-
- arginine
-
- ASS1
-
- argininosuccinate synthetase
-
- Cav
-
- canavanine
-
- СНX
-
- cycloheximide
-
- CM
-
- complete medium
-
- Isp
-
- indospicine
-
- PBS
-
- phosphate-buffered saline
-
- Rapa
-
- rapamycin
-
- rhARG
-
- recombinant human arginase I
Introduction
Genetic and metabolic alterations in many cancer cells make them more sensitive to the lack of certain exogenous amino acids (Sharma et al., 2019; Szefel et al., 2019; Zou et al., 2019). This provided a rational for the development of metabolic anticancer therapies based on the application of recombinant enzymes degrading individual amino acids, such as asparaginase for the treatment of pediatric leukemias (Covini et al., 2012; Beckett and Gervais, 2019; Ishihara et al., 2019; Tabe et al., 2019). Restriction of the semi-essential amino acid arginine (Arg) was proposed as a promising approach for the treatment of tumors deficient in Arg anabolism (Wheatley and Campbell, 2002; Wheatley, 2005). For some tumor types this therapy has already proceeded into clinical trials using two alternative recombinant Arg-depleting enzymes, human arginase I and arginine deiminase of bacterial origin (Ascierto et al., 2005; Glazer et al., 2010; Yau et al., 2013; Lowery et al., 2017; Abou-Alfa et al., 2018). However, Arg-deprivation therapy apparently has some limitations. One is unstable Arg auxotrophy in certain tumors in the course of the treatment due to restored expression of the ASS1 (argininosuccinate synthetase) gene, the key enzyme for Arg biosynthesis (Kurlishchuk et al., 2016; Alexandrou et al., 2018). It was also observed that the sensitivity of malignant cells to Arg deprivation as monotherapy is lower in clinical trials as well as in the three-dimensional (3D) tumor models as compared with classical monolayer cultures (Vynnytska-Myronovska et al., 2013; Tsai et al., 2017; Abou-Alfa et al., 2018). It was thus proposed that the combination of Arg deprivation with rationally selected chemotherapeutic agents could enhance its anticancer efficacy (Stasyk et al., 2015).
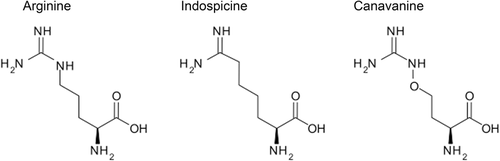
We have previously proposed the efficient combination modality of arginine deprivation with canavanine (Cav), a structural Arg analogue from leguminous plants (Vynnytska et al., 2011; Bobak et al., 2016; Kurlishchuk et al., 2016). In the present study, we addressed the therapeutic potential of another plant Arg analogue—indospicine (2(S)-2,7-diamino-7-iminoheptanoic acid, previously also described as l-6-amidino-2-aminohexanoic acid) (Netzel et al., 2019). Indospicine (Figure 1) has been studied as a non-protein amino acid toxic upon long-term exposure for grazing animals (Fletcher et al., 2015; Sultan et al., 2018). We speculated that this amino acid analogue may be specifically cytotoxic for Arg-deprived malignant cells, and concomitantly escape the degradation by a recombinant arginase. The data presented herein support these hypotheses.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), dialyzed FBS, gentamicin, l-arginine monohydrochloride (Arg), l-methionine, l-leucine, l-lysine monohydrochloride, rapamycin (Rapa), and cycloheximide (CHX) were purchased from Sigma-Aldrich (St. Louis, MO, USA). l-canavanine sulfate (Cav) was obtained from PAN Biotech GmbH (Aidenbach, Germany). Indospicine (Isp) was kindly provided by Associate Professor Fletcher M.T. Recombinant human arginase I (rhARG) used in this work was expressed as a secretory protein in the methylotrophic yeast Ogataea polymorpha and affinity-purified (our unpublished data).
Cell lines and culture conditions
Human colorectal carcinoma HCT-116, SW480, and SW620 as well as human immortalized keratinocyte cell lines HaCaT were obtained from the ATCC (Manassas, VA, USA). Cell cultures were routinely tested negative for mycoplasma contamination and were genetically validated at the Institute of Legal Medicine (TU Dresden, Germany) as previously detailed (Kurlishchuk et al., 2016) prior to use.
Cells were routinely incubated in DMEM supplemented with 50 mg/L gentamicin and 10% FBS at 37°C in a humidified atmosphere with 5% CO2. Arg deprivation was achieved by specially formulated DMEM (HyClone Laboratories, South Logan, UT, USA), lacking the amino acid Arg or by adding to the Arg-sufficient complete medium (CM) Arg degrading enzyme rhARG at a concentration of 2 U/mL. Arg-free medium (AFM) and the respective Arg-sufficient CM were supplemented with 10% dialyzed FBS devoid of small molecules including amino acids (<10 kDa cut off). Both means of selective Arg deprivation were shown previously to be identically effective in lowering intracellular Arg level and triggering cell cycle arrest in various tumor cell lines in vitro (Vynnytska-Myronovska et al., 2012, 2016; Hinrichs et al., 2018).
Assay of arginase activity
The activity of rhARG was assayed in phosphate-buffered saline (PBS) with 0.5 mM Arg and/or 0.5 mM Isp, or Cav and monitored via the level of urea production. For inhibition experiments, the reaction mixture was supplemented with 0.5 mM Arg analogues and pre-incubated for 2 h. After 1 h incubation of the reaction mixture at 37°C the resulting urea concentration was assessed spectrophotometrically at 520 nm after the addition of a color-producing reagent and heating at 100°C for 10 min, as described before (Marsh et al., 1965). The standard urea solution (Simko, Ukraine) and PBS were used as positive and negative controls, respectively.
Reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated using the GeneMATRIX Universal RNA Purification Kit (EURx, Gdansk, Poland) according to the manufacturer's instructions. Complementary DNA was synthesized from 2 μg of total RNA using the NG dART RT kit (EURx). Primer pairs for genes of interest were as follows: CHOP (forward, F: 5′-ATGGCAGCTGAGTCATTGCC-3′, reverse, R: 5′-AGCTGTGCCACTTTCCTTTC-3′); for GRP78 (Forward, F: 5′-TGTGCTCTCTGGTGATCAAG-3′, reverse, R: 5′-CGATTTCTTCAGGTGTCAGG-3′); MYC (forward, F: 5′-GGATTCTCTGCTCTCCTCGAC-3′, reverse, R: 5′-GCTGTGAGGAGGTTTGCTG-3′); ACTB (forward, F: 5′-TGCGTCTGGACCTGGCTG-3′, reverse, R: 5′-CTGCTGGAAGGTGGACAG-3′) was used as an internal control. PCR fragments were separated on 1.5% agarose gels and visualized by ethidium bromide staining. Relative intensity of the bands was quantified with Gel-Pro Analyzer version 3.2 and presented after normalization to the reference gene (ACTB).
Western blotting
Cells were homogenized on ice in lysis buffer as previously described (Vynnytska et al., 2011). Equal amounts of total proteins were resolved on 8–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane (Millipore Corporation, Bedford, MA, USA). Membranes were incubated with primary antibodies overnight at 4°C. Antibodies against the following antigens were used: phospho-Akt (Ser473) (#4060), Akt (#9272), phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (#14227), p44/42 MAPK (Erk1/2) (#4695), phospho-p38 MAPK (Thr180/Tyr182) (#9211), p38 MAPK (#9212), phospho-p70 S6 kinase (Thr389) (#9234), p70 S6 kinase (#2708), phospho-S6 ribosomal protein (Ser240/244) (#2215), S6 ribosomal protein (#2217), c-Myc (#5605), cleaved caspase-9 (Asp330) (#9501), cleaved caspase-3 (Asp175) (#9661), cleaved poly (ADP-ribose) polymerase (PARP) (Asp214) (#5625), all from Cell Signaling Technology, Danvers, MA, USA; GRP78 BiP (ab21685) from Abcam, Cambridge, UK; β-actin (A5441) from Sigma-Aldrich. After 1 h of exposure to horseradish peroxidase-conjugated goat anti-mouse (AP308P) or anti-rabbit (AP307P) IgG secondary antibodies (Millipore) at room temperature, immunoreactive proteins were visualized with Western Blotting Luminol Reagent (sc-2048; Santa Cruz Biotechnology, Heidelberg, Germany). All experiments were reproduced at least twice and the duplicate protein samples were subjected to a minimum of three independent western blot detections.
Cell viability detection by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
MTT reagent was purchased from Sigma-Aldrich. The assay was performed as described in our previous report (Vynnytska et al., 2011). Cell viability is presented in percent from the levels of the untreated controls at 0 h.
Statistical analysis
Mean values ± standard deviation from repeated experiments are shown and the number of independent experiments is indicated in the figure legends. Statistical analysis was performed using the Student's t test. Differences of the means were considered significant when the calculated P < 0.05.
Results
Indospicine does not inhibit arginase activity and is not an arginase substrate
It could not be excluded that Isp, due to its structural similarity to the proteinogenic amino acid arginine (Figure 1), may potentially inhibit arginase enzymatic activity, thus rendering a proposed combined treatment approach of Arg starvation with Isp inefficient. To address this question, we measured the specific activity of produced in yeasts rhARG in PBS in the absence or presence of Isp at equimolar to Arg concentration, as described in Materials and Methods. By monitoring urea production it was revealed that Isp at a 0.5 mM concentration had no significant inhibitory effect on rhARG activity even after previous pretreatment with Isp (Table 1). To the contrary, increased urea production was found when another Arg analogue Сav was present at the same concentration. This data also suggests, that, unlike Cav, Isp is not a catalytic substrate of rhARG.
| No. | Preincubation (2 h, 37°C) | Reaction (1 h, 37°C) | Urea level (mM) | % of control |
|---|---|---|---|---|
| 1 | rhARG + 0.5 mM Arg | 0.32 ± 0.03 | 100 | |
| 2 | rhARG + 0.5 mM Isp | nd | nd | |
| 3 | rhARG + 0.5 mM Arg + 0.5 mM Isp | 0.31 ± 0.02 (ns) | 97 | |
| 4 | rhARG + 0.5 mM Arg + 0.5 mM Cav | 0.44 ± 0.01* | 138 | |
| 5 | rhARG + 0.5 mM Isp | +0.5 mM Arg | 0.29 ± 0.03 (ns) | 91 |
| 6 | rhARG + 0.5 mM Cav | +0.5 mM Arg | 0.43 ± 0.02* | 134 |
- rhARG (2 U/mL) was incubated in PBS in the presence of 0.5 mM Arg or Isp alone (lines 1 and 2, respectively) or with Arg and co-administered Isp or Cav at equimolar concentrations without (lines 3 and 4, respectively) or with pretreatment (lines 5 and 6, respectively). Arginase activity was assessed by the level of urea production.
- Cav, canavanine; Isp, indospicine; nd, not detected; ns, non-significant; PBS, phosphate-buffered saline; rhARG, recombinant human arginase I.
- * P < 0.05 (vs. control value).
Arginine starvation profoundly increases indospicine cytotoxicity
We compared the cytotoxic effect of Isp in three human colorectal cancer (CRC) cell lines (HCT-116, SW480, and SW620) under Arg-rich and Arg-deficient culture conditions. For this purpose, the cells were treated with Isp at a broad range of concentrations (0, 0.025, 0.05, 0.1, and 1 mM) in both complete medium with 0.4 mM Arg (CM) and Arg-free medium (AFM). In addition, CM pre-incubated with rhARG (2 U/mL) for 24 h before cell exposure was applied as Arg-depleted condition. Cell viability was determined after 24, 48, and 72 h of incubation via the MTT assay. The amount of Isp required to kill 50% of the cells in culture was defined as IC50 (inhibitory concentration 50%).
No cytotoxic effect of Isp was observed in HCT-116 cells incubated in CM medium at concentrations of up to 1 mM. The same was true when cells were exposed to AFM for 24 h. However, at 48 h in AFM, Isp at concentrations of 0.025 mM led to 50% cell death in HCT-116 cultures; the effect became more pronounced in further time-course of the treatment. Accordingly, this Isp IC50 concentration for HCT-116 cells was applied in subsequent experiments (Figure 2A).
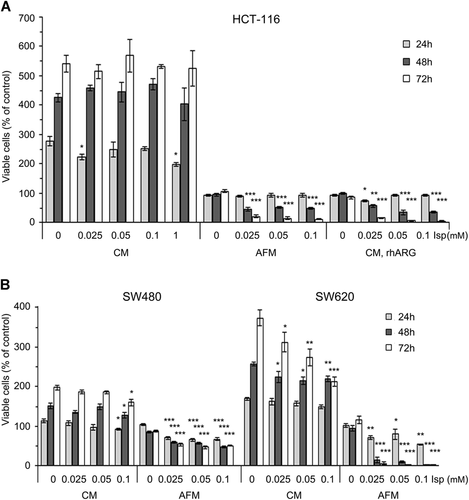
SW480 and SW620 colorectal carcinoma cells were also sensitive to the cytotoxic effect of Isp in AFM, much more pronounced for the latter cell line. In SW620 cultures, some dose-dependent decrease in the number of viable cells was observed even in Isp-supplemented CM (Figure 2B). Overall, the obtained results suggest that Arg deprivation profoundly sensitizes human colon cancer cells to the cytotoxic effects of Isp. However, non-transformed human keratinocyte HaCaT cells appeared to be much less sensitive to Isp action under Arg deprivation, exhibiting IC50 of 1 mM, that is, at least 40 times higher than all tested CRC cells (Figure S1A).
Impact of combined treatment of arginine deprivation and indospicine on cell signaling and apoptosis
HCT-116 cells were incubated in AFM alone or in AFM supplemented with 0.025 mM Isp for 24 and 72 h to evaluate the cumulative effects of Isp and Arg deprivation on cell signaling regulating survival and proliferative potential. For the analysis of MAPK and mammalian target of rapamycin (mTOR) pathways, c-Myc, Akt, markers of endoplasmic reticulum (ER) stress and apoptosis were assessed by western blot analysis and/or RT-PCR as described in the Materials and Methods section.
We first analyzed the level of phosphorylated Akt protein in Arg-starved HCT-116 cells in the presence and absence of Isp. Indeed, Arg deprivation alone caused a drastic increase in Akt phosphorylation in comparison to CM condition, whereas Isp treatment under AFM for 72 h resulted in a pronounced dephosphorylation of Akt and decrease in its protein level (Figure 3A).
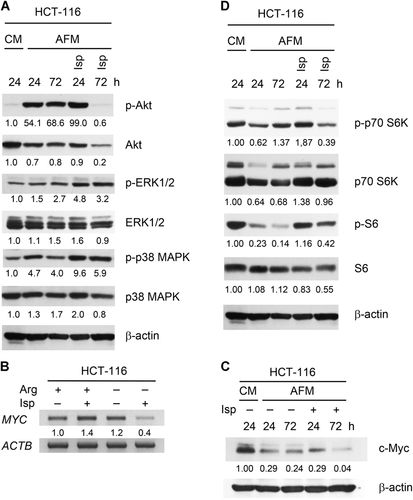
We also observed an increase in the phosphorylation of ERK1/2 and p38 MAPK proteins under arginine deprived conditions, which, in contrast to Akt, was further enhanced by Isp treatment (Figure 3A). Apparently, Isp evokes a strong activation of the MAPK/ERK axis in Arg-deprived cells.
The transcription factor c-Myc is often overexpressed in cancer cells and stimulates metabolic changes that are implicated in malignant transformation (Miller et al., 2012). We observed a pronounced specific decrease in c-Myc messenger RNA (mRNA) and protein levels in HCT-116 cells incubated for 72 h with Isp in AFM but not in cells exposed to AFM alone or CM + Isp, as demonstrated by semi-quantitative RT-PCR (Figure 3B) and western blot analyses (Figure 3C).
In line with previous reports (Vynnytska-Myronovska et al., 2016), long-term incubation of the cancer cells in AFM downregulated mTOR activity as evidenced by a more or less pronounced decrease in the phosphorylation levels of its downstream substrates p70S6 kinase (p70S6K) and S6 protein. This trend was reversed in the presence of Isp, indicating a transient activation of mTOR and of total protein translation in the cells by this Arg analogue (Figure 3D).
We reported previously that Arg starvation induces ER stress in solid cancer cells (Bobak et al., 2016). Therefore, ER stress markers GRP78 and CHOP were also monitored in Isp-treated HCT-116 cells. As expected, Arg deprivation alone significantly increased GRP78 and CHOP mRNAs relative to untreated cells. Co-treatment with Isp led to a certain increase in CHOP but not GRP78 expression (Figure 4A). However, western blot analysis revealed that GRP78 protein level also gradually increased at 72 h of the co-treatment (Figure 4B).
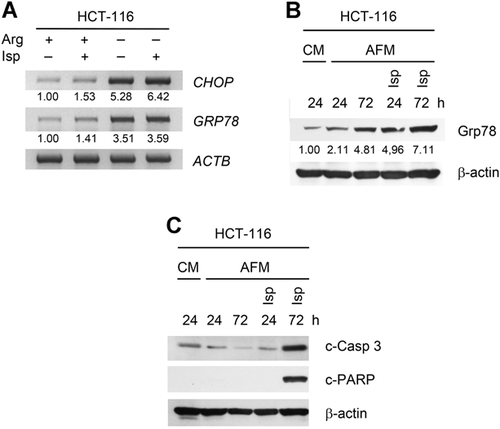
The alterations in PI3K-Akt-mTOR, MAPK/ERK signaling, as well as ER stress described above could directly affect apoptosis. Accordingly, HCT-116 cells treated with Isp in AFM for 72 h showed clear caspase 3 activation and PARP cleavage, whereas AFM mono-treatment did not induce apoptosis (Figure 4C). These results were essentially confirmed by cells Hoechst/PI staining demonstrating profound drop in the number of viable cells and induction of apoptosis under combined treatment with Isp (Figure S2).
The observed effects (Figures 3 and 4) tentatively indicate that Isp is misrecognized by cellular receptors and may be incorporated into nascent proteins in place of Arg, thus leading to the observed elevated ER stress and apoptosis.
Inhibition of protein synthesis rescues cells viability upon combined AFM and Isp treatment
To evaluate whether Isp is a proteinogenic amino acid in human cancer cells and whether this contributes to its cytotoxic effects, HCT-116 cells were incubated in AFM with or without Isp in the presence of the translation inhibitors rapamycin (Rapa, 0.04 µM; an inhibitor of mTOR signaling) and cycloheximide (CHX, 0.01 mM; inhibitor of peptidyltransferase). Cell viability was monitored after 72 h of incubation by the MTT test.
As shown in Figure 5A, both Rapa and CHX exhibited minor cytotoxic effects under AFM conditions, but at the same time significantly elevated cell viability in Isp-co-treated cells. The observed protective effect of CHX was concomitant with the inhibition of caspase-dependent apoptosis as evidenced by the decrease in the levels of cleaved forms of caspase-9, caspase-3, and PARP (Figure 5B).
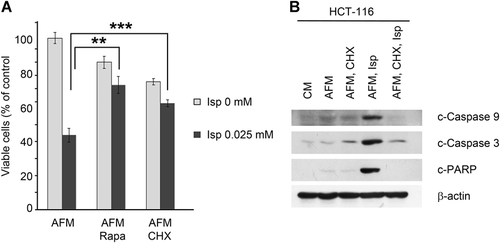
Thus, the observed cytotoxic effects of Isp towards Arg-deprived human CRC cells are most probably due to its misincorporation into nascent proteins, together with its impact on amino acid signaling.
Discussion
Although enzymatic Arg deprivation is considered as a nontoxic and selective anticancer therapy, it is not free of limitations. One limitation for this therapy to be effective is the requirement of Arg auxotrophy, ASS1 deficiency in particular, in the targeted tumors (Husson et al., 2003; Tsai et al., 2009; Bobak et al., 2010). Another one is reflected by the finding that cancerocidal potential of Arg starvation in traditional monolayer tumor cultures is apparently not fully translated into 3D cultures or in vivo conditions where it may lead to a pronounced but only transitional antiproliferative effect (Vynnytska-Myronovska et al., 2013, Tsai et al., 2017, Abou-Alfa et al., 2018). Rationally designed combination modalities based on Arg deprivation may overcome these drawbacks to some extent. We proposed and evaluated several combination approaches including the autophagy inhibitor chloroquine, the arginine proteomimetic toxic analogue Cav, and exogenous donor of nitric oxide as well as their triple combinations, which shall now be further tested in animal models (Vynnytska et al., 2011; Shuvayeva et al., 2014; Kurlishchuk et al., 2016; Mayevska et al., 2017). Other groups elaborated several similar or alternative approaches (Alexandrou et al., 2018; Harding et al., 2018; Hinrichs et al., 2018; Przystal et al., 2018).
Combination with Cav appeared to be very efficient in triggering ER stress and apoptosis in a broad range of the tested cancer cells, selective toward malignant cells in two-dimensional (2D) and 3D cultures, well-tolerated in murine models, and producing pronounced radiosensitizing effect (Vynnytska et al., 2011; Vynnytska-Myronovska et al., 2013; Bobak et al., 2016; Kurlishchuk et al., 2016; Vovk et al., 2016). However, we also found that only the combination of Cav with arginase but not with arginine deiminase as arginine degrading agent is feasible, since the latter enzyme efficiently degrades Cav (Vynnytska et al., 2011; and our unpublished results). Nonetheless, questions also remained on the Cav pharmacokinetics as this compound even at low micromolar concentrations is a weak catalytic substrate of rhARG and putative subject of its degradation (Vovk et al., 2016). We, therefore, searched for alternative Arg analogues such as the natural plant compounds, suitable and potentially superior to Cav as anticancer agents. Isp is a naturally occurring non-proteinogenic in the plant hosts amino acid, present in several species of the genus Indigofera that grow in certain regions of Australia and elsewhere in the world (Fletcher et al., 2015; Sultan et al., 2018). In contrast to Arg and Cav, Isp does not possess the guanidine group, which is recognized by arginase for hydrolysis (Figure 1). Isp has been reported to be toxic for grazing animals and domestic animals consuming their meat which contains Isp accumulated as the free amino acid (Tan et al., 2016; Netzel et al., 2019).
To check the suitability of Isp for combination therapy with arginase, we examined whether Isp is able to inhibit the enzyme by competing with Arg for binding at the catalytic center. Our data revealed that Isp at 0.5 mM concentration had no detectable inhibitory effect on rhARG activity at physiological pH in the presence of equimolar concentrations of Arg. It was also demonstrated that Isp is not a catalytic substrate of rhARG (Table 1). As previously reported, Isp inhibited arginase activity in rat liver homogenate in vitro (Madsen and Hegarty, 1970). It should be noted, however, that a dose-dependent inhibition was observed in this early work at significantly higher (by one order of magnitude or greater) concentration in comparison to Isp IC50 concentration of 0.025 mM used in the present study. Thus, Isp, as a compound not interfering with rhARG, can potentially be applied in combination with this Arg-degrading enzyme for metabolic therapy.
We evaluated the cytotoxic effect of Isp under Arg deprivation in three CRC human line models HCT-116, SW480, and SW620, exhibiting variable expression patterns of ASS1 (Kurlishchuk et al., 2016; Bateman et al., 2017; Alexandrou et al., 2018). The data presented herein indicate that the anti-cancer effect of Isp is analogous to or even stronger than that of Cav under the same conditions (Vynnytska et al., 2011). We have recently shown that Arg starvation activates targets of prosurvival pathways in CRC cells, that is, PI3K-Akt, p38MAPK, and ERK1/2 (Bobak et al., 2016), and therefore further elucidated signaling pathways that may mediate the cytotoxic effects of Isp upon Arg deprivation. Isp co-administration for 72 h caused deactivation of PI3K-Akt pathway and, unexpectedly, overactivation of MAPK-related pathways. Of note, it is known that prolonged ERK/MAPK activation leads to cancer cell death through growth arrest and apoptosis (Cagnol and Chambard, 2010; Pathania et al., 2014).
We also observed that the lack of Arg in HCT-116 cells does not profoundly affect the expression of MYC, a master regulator of cellular metabolism and proliferation, overexpressed in many human cancers (Dang et al., 2009; Miller et al., 2012). However, concomitant Isp treatment led to a drastic decrease in gene expression and c-Myc protein level which may negatively affect cell survival (Safe et al., 2018).
It is known that starvation for certain amino acids induces translation arrest and abrogates proliferation. This regulation is mediated by mTOR signaling (Saxton and Sabatini, 2017). We here observed that Isp effectively reverses the Arg deprivation dependent inactivation of the mTOR pathway, as evidenced by the increased phosphorylation of p70S6K and its substrate S6 in co-treated HCT-116 cells. This data suggests that Isp most probably mimics Arg in the regulation of mTOR.
Arg deprivation was reported earlier to induce ER stress in HCT-116 cells reflected by the abundance of mRNAs of the ER stress markers—proapoptotic CHOP and ER chaperon GRP78 (Bobak et al., 2016). In the present study, Isp co-treatment was found to further significantly upregulate these genes, thus behaving similarly to Cav (Bobak et al., 2016). Chronic or unresolved ER stress often results in the induction of apoptosis (Gorman et al., 2012). Indeed, exposure to Isp for 72 h under Arg starvation evoked profound activation of caspase-3-dependent apoptosis in the CRC model.
It was demonstrated previously that the inhibitor of protein synthesis CHX rescues the viability of Arg-deprived cancer cells co-treated with Cav, thus indicating that toxic effects of this analogue are due to its aberrant incorporation into newly synthesized proteins (Vynnytska et al., 2011). Older publications also suggested that Isp is not a proteinogenic amino acid due to its inhibition of arginyl-transfer RNA (tRNA) synthetase (Madsen et al., 1970). However, inhibition of arginyl-tRNA formation in vitro was observed at Isp concentrations of 1 mM and higher (Madsen et al., 1970). In our experiments, both an inhibitor of translation at the stage of mTOR signaling (Rapa) and an inhibitor of translation elongation (CHX) effectively protected Arg-starved cells from Isp-induced caspase-dependent apoptosis and elevated their viability. We therefore propose that, when a competitor molecule is eliminated under Arg deprivation conditions, Isp even at low concentrations behaves similar to Cav (Vynnytska et al., 2011), that is, is incorporated into nascent proteins, structurally and functionally compromising them. This notion has to be experimentally substantiated in the follow up studies. Although all tested signaling pathways act in concert, it is believed that aberrant activation of mTOR and protein translation by Isp may be one of the primary causes of subsequent exacerbation of ER stress and apoptosis under combination treatment.
In summary, we report for the first time that Isp, an Arg analogue of plant origin, at low concentrations in combination with Arg-deprivation is very cytotoxic toward model CRC cells clearly leading to a synergic effect. We also demonstrate that this compound is neither an rhARG substrate nor its inhibitor. In Arg-deprived CRC cells Isp efficiently up-induces ER stress, several anti-proliferative and pro-apoptotic pathways and caspase-dependent apoptosis. We propose that the cytotoxic effects of Isp most likely result from its incorporation into nascent cellular proteins as judged from translation inhibition experiments. Future comparative studies on alternative cellular and, most importantly, in vivo models are envisioned to clarify, which Arg analogue may be superior for co-application with Arg-deprivation therapy.
Acknowledgment and funding
The authors appreciate the technical support of Dr. Y. Kurlishchuk and O. Kluchivska in executing some experiments described in this work. The work was supported in part by the grant of the State Fund for Fundamental Research of Ukraine F76/51-2018.
Conflict of interest
The authors declare no conflict of interest.




