Extracellular laccase from Monilinia fructicola: isolation, primary characterization and application
Abstract
Laccases are enzymes belonging to the family of blue copper oxidases. Due to their broad substrate specificity, they are widely used in many industrial processes and environmental bioremediations for removal of a large number of pollutants. During last decades, laccases attracted scientific interest also as highly promising enzymes to be used in bioanalytics. The aim of this study is to obtain a highly purified laccase from an efficient fungal producer and to demonstrate the applicability of this enzyme for analytics and bioremediation. To select the best microbial source of laccase, a screening of fungal strains was carried out and the fungus Monilinia fructicola was chosen as a producer of an extracellular enzyme. Optimal cultivation conditions for the highest yield of laccase were established; the enzyme was purified by a column chromatography and partially characterized. Molecular mass of the laccase subunit was determined to be near 35 kDa; the optimal pH ranges for the highest activity and stability are 4.5–5.0 and 3.0–5.0, respectively; the optimal temperature for laccase activity is 30°C. Laccase preparation was successfully used as a biocatalyst in the amperometric biosensor for bisphenol A assay and in the bioreactor for bioremediation of some xenobiotics.
Abbreviations
-
- AB
-
- acetate buffer
-
- ABS
-
- amperometric biosensor
-
- ABTS
-
- 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonate)
-
- BP
-
- bisphenol A
-
- CL
-
- cultural liquid
-
- DF
-
- diclofenac
-
- DTT
-
- dithiothreitol
-
- LA
-
- laccase activity
-
- MM
-
- mineral medium
-
- SA
-
- specific activity
-
- SDS
-
- sodium dodecyl sulfate
Introduction
Laccases (EC 1.10.3.2) are a family of copper-containing oxidases found in a variety of bacteria, fungi, insects, and plants (Baldrian, 2006; Kunamneni et al., 2008; Forootanfar et al., 2011; Martínez et al., 2017). A lot of fungi, ascomycetes, basidiomycetes, and deuteromycetes can produce laccases, but the most efficient laccase producers are white-rot basidiomycetes (Myasoedova et al., 2017; Patel et al., 2019; Pereira-Patrón et al., 2019). Until now, more than 100 laccases have been isolated from the fungal cultures and characterized (Baldrian, 2006; Morozova et al., 2007; Chakroun et al., 2010; Sitarz et al., 2016; Glazunova et al., 2019; Patel et al., 2019). Laccases were found in soil and some freshwater Ascomycetes species (Baldrian, 2006). A lot of laccase-producing recombinant strains were engineered, combining rational and computational design with directed evolution, to attain the desirable catalytic efficiency and stability properties required for laccase's industrial utilization (Rivera-Hoyos et al., 2015; Antošová and Sychrová, 2016; Martínez et al., 2017; Herkommerová et al., 2018).
Laccases have a broad substrate specificity, oxidizing mono-, di-, poly-, and methoxy-phenols, aromatic and aliphatic amines, hydroxyindoles, benzenethiols, carbohydrates, and inorganic/organic metal compounds. Fungal laccases are implicated in both intracellular and extracellular physiological processes including delignification, morphogenesis, pigmentation, and pathogenesis (Baldrian, 2006; Forootanfar et al., 2011; Mate and Alcalde, 2017; Patel et al., 2019).
Laccase production is dependent on a type of cultivation, composition of growth medium, and different inducers (Baldrian, 2006; Mate and Alcalde, 2017; Patel et al., 2019). Glycosylation content and composition of glycoprotein vary with growth medium composition (Glazunova et al., 2019). Copper ions in a low concentration are the most used inducer in laccase production (Yang et al., 2017; Patel et al., 2019). In blue copper oxidases, the Cu2+-binding domains are highly conserved. Glycosylation plays an important role in copper retention, thermal stability, susceptibility to proteolytic degradation, and secretion. Besides copper, manganese and zinc are also commonly found to stimulate laccase synthesis (Gomaa and Momtaz, 2015; Yang et al., 2017). Laccases are commonly monomeric, di-, three- and tetrameric glycoproteins with molecular weight ranges from about 50 up to 110 kDa (Baldrian, 2006; Morozova et al., 2007; Kaczmarek et al., 2017).
The effect of organic compounds, including xenobiotics, on laccase production depends on the compound structure, fungal strain, growth stage, laccase isozyme as well as the culture medium (Yang et al., 2017; Gupta and Jana, 2019; Patel et al., 2019). Combinational induction of laccase production by metal ions and organic compounds can be either synergistic (Yang et al., 2017) or antagonistic (Zhuo et al., 2017). Li et al. (2011) found that glycerol produced from glucose by the yeast Candida sp. is an efficient carbon source for Ganoderma lucidum upon glucose deprivation and crucial for laccase overproduction by prolonging laccase secretion time (Li et al., 2011). Phenolics and lysing enzymes produced by opposing microbes have also been suggested to have laccase-inducing ability (Yang et al., 2017).
Simple and efficient schemes for laccase purification from natural and recombinant producers were described, including protein precipitation by ammonium sulfate or ethanol, column chromatography on ion-exchange or hydrophobic sorbents, gel filtration, desalt/buffer exchange of protein, and gel filtration chromatography (Morozova et al., 2007; Chakroun et al., 2010; Patel et al., 2019; Savinova et al., 2019). Upon purification, laccase enzymes demonstrate considerable heterogeneity (Savinova et al., 2019). Laccases from different fungal strains was shown to vary in properties. It was estimated recently that N-linked glycosylation plays a substantial role in moderation of enzymatic properties of laccases (Glazunova et al., 2019).
Laccases are able to oxidize phenolic and non-phenolic lignin-related compounds as well as highly recalcitrant environmental pollutants. This makes these enzymes very useful for their application in several biotechnological processes, namely in the bioremediation of contaminated soils, detoxification of industrial effluents, mostly from the paper and pulp, in the pharmaceutical, chemical, food, textile, petrochemical, and wood processing industries (Lin et al., 2016; Upadhyay et al., 2016; Wang and Wang, 2016; Rani et al., 2017; Yang et al., 2017; Zeng et al., 2017; Patel et al., 2019; Xueping and Shikeren, 2019). Laccases are also used as biocatalysts in polymer synthesis (Khlupova et al., 2015; Roth and Spiess, 2015; Su et al., 2018; Khlupova et al., 2019; Vasil'eva et al., 2019; Walde et al., 2019), for wine and beverage stabilization (Claus and Mojsov, 2018), for the manufacture of anti-cancer drugs and as ingredients in cosmetics (Kunamneni et al., 2008). Recently, the utility of laccases has also been applied to nanobiotechnology (Mate and Alcalde, 2017; Rani et al., 2017; Naghdi et al., 2019).
Commercially, laccases have been used for differential testing of codeine and morphine, to delignify woody tissues in production of ethanol (Patel et al., 2019). In biofuel production laccase has significant potential use not only as a delignifying enzyme, but also as an effective biocatalyst for removal of phenolic inhibitors of subsequent enzymatic processes (Kudanga and Le Roes-Hill, 2014).
Laccase, being multi-copper oxidase with simple structure and specific function, is a promising biocatalyst for bioreactors and electrochemical devices—biosensors and enzymatic biofuel cells. The enzyme play a special role as biorecognition element in biosensors for detection of toxic pollutants of phenolic nature (Rodríguez-Delgado et al., 2015; Yashas et al., 2018; Zhang et al., 2018; Campaсa et al., 2019; Kavetskyy et al., 2019; Mohtar et al., 2019; Patel et al., 2019; Zhang et al., 2020) and designing of biofuel cells (Kudanga and Le Roes-Hill, 2014; Kang et al., 2018; Ghosh et al., 2019; Han et al., 2019; Zerva et al., 2019; Zhang et al., 2019).
Phenols as the main substrates of laccase usually are detected with amperometric biosensors (ABS) or degraded with laccase-based bioreactors (Karim and Fakhruddin, 2012; Xueping and Shikeren, 2019; Zerva et al., 2019). To immobilize laccase for development of both devices, biosensors and bioreactors, a variety of methods (adsorption, covalent binding, entrapment, cross-linking, etc.) and a broad number of carriers (polymeric and nanocomposites) are used (Karim and Fakhruddin, 2012; Kang et al., 2018; Castrovilli et al., 2019; Fu et al., 2019; Han et al., 2019; Zerva et al., 2019).
During the last decade, different molecular engineering methods to create highly efficient recombinant producers of laccases were proposed. Recombinant enzymes with improved properties as efficient and tailored catalysts for specific applications in scientific investigations, biotechnological, and industrial exploitation were purified and characterized (Stanzione et al., 2020). However, classical screening and selection of promising laccase microbial producers from nature, followed by optimization of culture conditions, still constitute a viable and effective approach to isolate organisms with tremendous laccase synthesis ability.
The aim of our study is to obtain a highly purified laccase from an efficient fungal producer Monilinia fructicola and to demonstrate the applicability of this enzyme for analytics and bioremediation.
Materials and methods
Chemicals
Sodium dodecyl sulfate (SDS), 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonate) (ABTS), dithiothreitol (DTT), chitosane, bisphenol A (BP) and diclofenac (DF), inorganic salts, and other chemicals were obtained from Sigma-Aldrich and Fluka unless mentioned otherwise.
Strains
The following fungal strains were used for the selection of the most effective producer of extracellular laccase: Botrytis allii from microbial collection of Institute of Cell Biology National Academy of Sciences of Ukraine, Botrytis aclada, M. fructicola, Sporobolomyces salmonicolor, Torula sp, Stachybotris chartarum, Aureobasidium pullulans, Absidia glauca, Fusarium oxysporum, Сhaetomium globosum from Rzeszow University, Poland; Trichothocium roseum, Oidium lactis, Trichoderma lignorum, Aspergillus oryzea, Fusarium sp., Penicillum chrysogenum from Ivan Franko Lviv National University.
Cultivation of fungal cells
The cultivation mineral medium (MM) used for testing laccase production was composed as follows (g/L): KH2PO4, 2.5; NaNO3, 2.5; MgSO4·7H2O, 0.5; NaCl, 0.5; glucose, 20.0; yeast extract, 0.2. Rich medium contains (g/L): yeast extract, 2; pepton, 4; glucose, 15. Both mediums were supplemented with trace elements solution containing (mg/mL): Na2B4O7·10H2O, 0.1; CuSO4·5H2O, 0.01; FeSO4·7H2O, 0.05; MnSO4·7H2O, 0.01; ZnSO4·7H2O, 0.07; (NH4)6Mo7O24·4H2O, 0.01. After boiling and filtration, pH of MM was adjusted to 5.5. The influence of carbon sources on yeast growth and laccase activity (LA) was studied using 1.5% glycerol, 1% maltose or 1% ethanol, 1% glucose, 1% mannose, 1% fructose, and 1% lactose.
To study the effect of nitrogen sources on laccase production, ammonium sulfate, malt and yeast extracts, urea, and pepton (0.3%) were used.
Enzyme characterization
The activity of laccase was determined by the rate of the increase in absorbance monitored spectrophotometrically at 420 nm (Shimadzu, Japan). As a substrate, 0.5 mM ABTS in 50 mM sodium acetate buffer solution, pH 4.5 was used. One unit of LA was defined as the amount of the enzyme required to oxidize 1 µmole of a substrate (ε420 = 36 mM-1cm-1) per minute at 24°C. The estimation of protein content in the enzyme solution was performed according to Lowry.
The optimal temperature was tested in the range from 20 to 90°C. The thermal stability was measured after enzyme pre-incubation under different temperatures for 1 h followed by determination of activities under standard conditions. The optimal pH value and the pH stability were determined in 50 mM acetate buffer (pH 3.0–6.0), 50 mM sodium phosphate buffer (6.0–8.0), and 50 mM glycine-NaOH buffer (pH 9.0–12.0). The pH stability was tested after enzyme's pre-incubation at different pH during 1 h at 24°C.
To study the effect of some chemical compounds on the LA, the tested substances were mixed with enzyme solution and pre-incubated at 24°C for 1 h before determination of LA. The tested additives were as follows: 1 and 10 mM CdCl2, CoCl2, CrCl3, CuCl2, FeCl2, FeCl3, MnCl2, NiCl2, HgCl2, ZnCl2, AgNO3, Pb(NO3)2, and ethylenediaminetetraacetic acid, 2 and 5 mM SDS, 2 mM DTT, 2 mM sodium azide as well as different organic solvents (5, 20, and 50%).
Isolation and purification of laccase from extracellular medium
The enzyme was isolated from a cultural liquid (CL) of the fungus M. fructicola. For this aim, the cells were cultivated at 28°С in MM as applied to CM-Sephadex 25 column, equilibrated with 50 mM Na acetate buffer, pH 4.5 (AB) and washed with the same buffer. The purification was carried out at the room temperature. Unbound proteins containing LA were loaded on DEAE-Toyopearl M-650 column equilibrated with AB. The column was washed with AB, the bound proteins were eluted stepwise with 0.1–0.5 M NaCl in AB. Fractions with the LA were pooled, concentrated by Millipore filter, 10 kDa, and finally lyophilized. Electrophoretic analysis of the protein fractions was performed in 12% SDS–PAAG as described by Laemmli; protein bands were visualized by Coomassie brilliant blue R-250 staining.
Biosensor construction
The ABS was evaluated using constant potential amperometry in a three-electrode configuration with an Ag/AgCl/KCl (3 M) reference electrode, a Pt-wire counter electrode, and a working platinum electrode. Amperometric measurements were carried out using a potentiostat CHI 1200A (IJ Cambria Scientific, Burry Port, UK) connected to a personal computer and performed in a batch mode under continuous stirring in an electrochemical cell with a 20 mL volume at 25°C.
The construction of bioelectrode was performed as follows. Platinum electrode (0.71 cm2) was covered by polianiline layer using electropolimerization (Stasyuk et al., 2012). On a rinsed surface, the laccase solution (200 U/mL) was dropped and dried. Finally, neutralized Nafion solution was added to form the outer membrane covering. The obtained bioelectrode was rinsed by 30 mM AB, pH 5.0 and kept at +4°C before usage.
Bioreactor construction and investigation
For construction of laboratory prototype of laccase-based bioreactor, laссase was entrapped into activated by glutaric aldehyde chitozan as described by (Zheng et al., 2016). The laccase solution in 5 mM AB, pH 5.0 was mixed with the activated chitozan beads. The obtained laccase/chitizan beads were packed in the column-type tubes and used as bioreactor in the experiments for bioremediation of BP and DF in model solutions.
Concentrations of BP and DF during degradation in laccase-based bioreactor were measured by high-performance liquid chromatography (HPLC) (Dionex UltiMate 3000) with ultraviolet (UV) detection (Dionex UltiMate 3000 RS Variable Wavelength Detector). Separation of BP and DF (20 µL) was performed on a ACE 5 C18 column (5 µm, 250 × 4.6 mm), with guard column, using the mobile phase 2 mM acetic acid:methanol (30:70, v/v), which were detected by UV at 274 nm.
Acquisition and integration of chromatograms was performed using the Chromeleon Chromatography Data System 6.8 software.
Statistical analysis
Statistical analysis was carried out using SPSS 20.0 software. Results obtained from the experimentation were expressed as mean values ± standard error of the means, n = 3. A probability of P < 0.05 or 0.01 (*P < 0.05 or **P < 0.01) was interpreted as significant or highly significant, respectively.
Results and discussion
Selection of an efficient fungal producer of an extracellular laccase and optimization of cultivation conditions
To choose the best producer of laccase, a screening of various fungal strains (Strains section) was carried out. For this aim, different fungi were cultivated for 3 days in MM, containing 1% glucose and 0.05% yeast extract, with daily monitoring of extracellular LA. Among the tested strains, LA was revealed in M. fructicola, S. chartarum, and B. aclada (Demkiv et al., 2016). The fungus M. fructicola on the third day of cultivation showed the highest specific LA, namely 13.8-fold higher than S. chartarum and 2.3-fold higher than B. aclada. Thus, fungus was chosen as the best producer of extracellular laccase.
Carbon and nitrogen sources, as well as some transient metal ions as inducers, are the most critical factors that influence the laccase synthesis. Glucose, mannose, maltose, fructose, and lactose are the commonly used carbon sources, while yeast extract, peptone, urea, (NH4)2SO4, and NaNO3 are traditional nitrogen sources. Laccase production is triggered by nitrogen depletion, but some nitrogen sources do not affect the enzyme activity. Some works show that the elevated LA was achieved by using a low carbon-to-nitrogen ratio, while others recommend a high carbon-to-nitrogen ratio (Wang et al., 2014; Patel et al., 2019). Aromatic compounds and some inorganic ions were shown to be inducers of LA (Baldrian, 2006; Patel et al., 2019).
For fungus M. fructicola, the effect of cultivation conditions (composition of the medium and cultivation time) on LA was studied. The influence of different carbon sources, present in the medium, on enzymatic activity is shown in Figure 1A. LA is highest under cultivation with maltose, and 1.6-fold lower LA was observed under cultivation with glucose, sucrose, and xylitol (Figure 1A). Figure 1B demonstrates the influence of carbon sources on LA in more detail, namely, as an effect of cultivation time. The highest LA was shown to be achieved on the fifth day of cultivation with maltose. This value is twofold higher compared with glucose or xylitol. The data on Figure 1C demonstrates that ammonium sulfate is the most efficient source of nitrogen among the tested ones. The effect of cultivation temperature (25, 28, and 35°С) and its duration on LA was studied (Figure 1D). The highest LA was observed under cultivation of cells at 28°С (180 mU/mL). This value is 1.16-fold higher in comparison with correspondent activity at 25°С (154 mU/mL) on fifth day of cultivation. At 35°С, M. fructicola cells do not grow, so LA under these conditions was not tested.
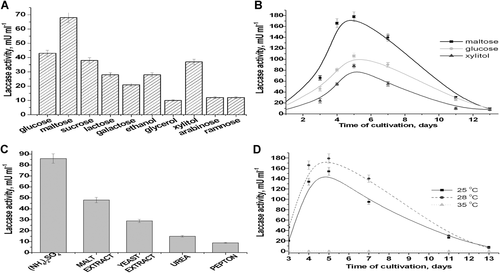
From the literature, it is known that the optimal temperature for laccase production differs for different strains, varying in the range between 25 and 30°C (Patel et al., 2019). In our experiments, we tested the temperature effect at 25, 28, and 35°C (Figure 1). The highest LA for M. fructicola is achieved under fhe following optimal composition of MM: maltose as a carbon source, ammonium sulfate as a nitrogen source, and cultivation at 28°С during 5 days.
Effect of different metal ions as the inducers of laccase synthesis was investigated. In our previous experiments, we have studied the effect of different concentrations (from 0 up to 1 mМ) of Cu2+ ions on LA under cultivation in optimal MM. The most effective inducer of M. fructicola laccase was shown to be 0.5 mM Cu2+ ions. These results coincide with the literature that Cu2+ ions take part in regulation of laccase-coding gene transcription (Wang et al., 2014). The influence of other metal ions on LA activity during cultivation in the rich growh medium was studied in more detail (Figure 2). The salts were added to the fungal cells after 2 days of cultivation.
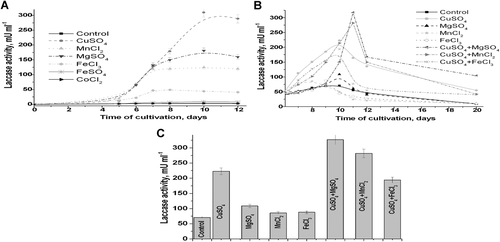
It was demonstrated (Figure 2) that in the presence of Cu2+, Mg2+, Mn2+, Fe2+, as well as of mixtures Mg2+/Cu2+, Mn2+/Cu2+, and Fe2+/Cu2+ in the growth medium, the extracellular LA were increased 3.3-, 1.6-, 1.3-, 1.2-, 5-, 4.2-, 2.9-folds, respectively. The stimulatory effect of Cu2+ on laccase production is also described for several other fungi and could be used as a simple method to improve the enzyme production. Besides copper, manganese ions are also commonly found to stimulate laccase synthesis (Jarosz-Wilkołazka et al., 2013; Yang et al., 2017; Zhuo et al., 2017).
Isolation, purification, and characterization of extracellular laccase M. fructicola
Purification of the enzyme from a 5-day extracellular liquid was performed by a two-step column chromatography. Specific activity (SA) of laccase in each fraction was calculated. According to SA, 3.5-fold laccase purification was achived in the fraction of unbound proteins on cation exchange sorbent СМ-Sephadex С-25. On the next sorbent, anion exchange DEAE-Toypearl 650 M, 11.6-fold purification was achieved in eluates. The active fractions of laccase were finally concentrated by microfiltration. As a result, 20-fold purified enzyme with 30% yield and SA 3.45 units per mg of protein was obtained. It was shown that after liophilization the enzyme kept about 90% of its activity. Characteristics of chromatographic purification of laccase are presented in Table 1.
| Activity | |||||
|---|---|---|---|---|---|
| Stage | U ·ml-1 | U mg-1protein | Total (U) | Yield (%) | Purification Factor |
| Extracellular liquid, 1 L | 0.064 | 0.19 | 64.0 | 100 | 1 |
| Sephadex СM-25, unbound proteins fraction | 0.078 | 0.65 | 57.6 | 90 | 3.5 |
| DEAE-Toypearl 650M, eluate by 1 M NaCl | 0.145 | 2.4 | 24.0 | 38 | 11.6 |
| Concentration on Millipore filter, 10 kDa | 0.150 | 3.75 | 19.2 | 30 | 19.9 |
The purity of the isolated enzyme preparation was approved by SDS-polyacrylamide gel electrophoresis (SDS-PAAG) (Figure 3A). The molecular weights of the laccase subunits were calculated from the electrophoretic mobility of the protein and a set of standard proteins (Figure 3B).
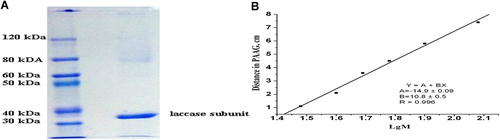
Some physico-chemical characteristics of the purified laccase in comparison with the described laccase preparations are shown in Table 2. Laccase monomers vary in molecular mass from 43 to 383 kDa, but typical fungal laccases have a molecular mass of 60–70 kDa. For M. fructicola laccase, Michaelis–Menten constant (KM) estimated toward ABTS is 0.013 mМ. The optimal conditions for the highest activity of the purified laccase were estimated with ABTS as a substrate. The pH optimum is in range 4.5–5.0. The most storage stability is under pH 3.0–5.0. The optimal temperature for LA is 28°C. The enzyme is stable at the temperatures from 20 to 45°C during 60 min, but it loses 25% of activity at 55°С. These characteristics are similar to the properties of the known laccases from other organisms (Baldrian, 2006; Yang et al., 2017; Patel et al., 2019).
| Source | Molecular mass of subunit (kDa) | Optimal pH | Optimal temperature (°C) | КM for ABTS (mM) | Reference |
|---|---|---|---|---|---|
| M. fructicola | 35 | 4.0 | 30 | 0.013 | The current paper |
| Monilinia fructigena | 77.6 | 3.5 | 45 | 0.012 | Chakroun et al, 2010 |
| Thielavia sp. | 70 | 5.0 | 0.023 | Mtibaà et al, 2018 | |
| Trichoderma atroviride | 80 | 3 | 50 | 2.5 | Chakroun et al, 2010 |
| Paraconiothyrium variabile | 84 | 4.8 | 50 | 0.203 | Chakroun et al, 2010 |
| Cladosporium cladosporiodes | 71.2 | 3.5 | 40–70 | – | Baldrian 2006 |
| Trametes versicolor 951022 | 97 | 3.0 | 50 | 0.013 | Chakroun et al, 2010 |
| Trametes sp | 62 | 4.5 | 50 | 0.025 | Chakroun et al, 2010 |
According to the literature, the optimal temperature for LA is ranging from 25 to 80°C, although the thermostability of fungal laccases varies considerably. The half-life at 50°C ranges from minutes for laccase from B. cinnerea to over 3 h—for Lentinus edodes and Agaricus bisporus, and up to 70 h for Trametes species. Laccase from G. lucidum is immediately inactivated at 60°C, while the thermostable laccase from Melanocarpus albomyces exhibits a half-life over 5 h (Baldrian, 2006; Chakroun et al., 2010; Patel et al., 2019).
Some physicochemical characteristics of the purified M. fructicola laccase in comparison with the described laccase preparations are shown in Table 2. Laccase monomers vary in molecular mass from 43 to 383 kDa, but typical fungal laccases have a molecular mass of 60–70 kDa. For laccase from M. fructicola, KM, estimated toward ABTS, is 0.013 mМ. The optimal conditions for the highest activity of the purified laccase were estimated with ABTS as a substrate. The pH optimum is in range 4.5–5.0. The most storage stability is under pH 3.0–5.0. The optimal temperature for LA is 28°C. The enzyme is stable at the temperatures from 20 to 45°C during 60 min, but it loses 25% of activity at 55°С. These characteristics are similar to the properties of the known laccases from other microorganisms (Baldrian, 2006).
Heavy metal ions are common environmental pollutants and can affect the production and stability of the extracellular enzymes (Baldrian, 2006; Jarosz-Wilkolazka et al., 2013; Yang et al., 2017; Zhuo et al., 2017; Patel et al., 2019). The effect of heavy metal ions on LA was tested by using ABTS as the substrate. It was shown that 1 mM Cd2+, Zn2+, Fe3+, Mg2+, Ag+, Cu2+, Co2+, Mg2+, and Cu2+ have inducible effect on LA, but under increased concentration of salts (10 mM), only Mg2+ and Cu2+ ions are inducers of enzyme activity. A lot of the studies have shown that laccase is a copper-containing oxidase, so a low level of Cu2+ resulted in increasing enzyme activity (Baldrian, 2006; Yang et al., 2017; Zhuo et al., 2017; Patel et al., 2019). Sodium azide and SDS were shown to be the inhibitors of LA.
Construction of the laccase-based bioelectrode for analysis of BP
Bisphenols are widespread chemical compounds in modern life because of their industrial application as plasticizers in the synthesis of polycarbonate (PC) plastics and epoxy resins. BP is the best-known member of these endocrine disrupting chemicals (Mikołajewska et al., 2015). Exposure to BP was shown to occur through the consumption of beverages or foods that have been in contact with PC plastic containers or epoxy resins in food packaging (Kubwabo et al., 2009).
BP has dangerous influence on the child's organism. Prenatal exposure to maternal BP concentrations was related to higher levels of anxiety, depression, aggression, and hyperactivity in children. The exposure to BP in childhood adds to listed problems other ones, namely, higher levels of inattention and problems in the child's behavior (Hoekstra and Simoneau, 2013; Lee et al., 2014; Ejaredar et al., 2017).
That is why the problem of adequate evaluation of BP level in water and food products is necessary. Thus, for human safety the development of simple sensitive selective methods for BP analysis, including enzyme biosensors, is very actual.
A limited number of reports describe the application of enzyme biosensors for BP analysis. As biorecognition elements in such biosensors, laccase, tyrosinase, peroxidase, and other enzymes are used (Karim and Fakhruddin, 2012; Ragavan et al., 2013; Kochana et al., 2015; Rodríguez-Delgado et al., 2015; Zerva et al., 2019; Gugoasa, 2020).
Enzyme-based biosensors operate at lower potential values than chemosensors and as a result, these biosensors have a higher selectivity to key analytes. As immobilization matrixes for laccase-based biosensors and bioreactors, carbon nanomaterials (Liu et al., 2007), metal nanoparticles (Chen et al., 2015), metal oxides (Chawla et al., 2012), electroconductive polymers (Rahman et al., 2008), and ionic liquids (Franzoi et al., 2009) are successfully used. The peculiarity of nanocomposite materials as carriers for laccase immobilization is their large specific surface area and good biocompatibility, which resulted in improved sensitivity, good selectivity, stability, repeatability, and reproducibility of biosensors (Castrovilli et al., 2019; Zerva et al., 2019; Zhang et al., 2020). In particular, electrode construction with carbon nanotubes, fullerene (C60), graphene, and other carbon materials to increase surface area and conductivity has been attempted (Dong et al., 2017).
In our investigation, we describe the construction of the bioelectrode based on laccase, immobilized in polyaniline matrix on the surface of the Pt electrode (laccase/PANi bioelectrode). Figure 4 shows a chronoamperogramm of the proposed laccase/PANi bioelectrode. An optimal working potential of this process, which corresponds to −100 mV versus Ag/AgCl, was chosen experimentally.
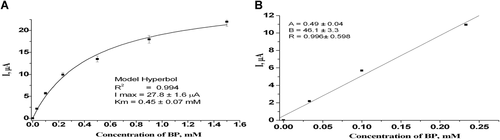
Following to the chronoamperogramm of the bioelectrode obtained, a calibration curve of responses on the increasing concentrations of BP was plotted as illustrated in Figure 4A. As seen from the calibration curve, a biosensor response Imax was estimated to be 27.8 μA (at electrode surface 0.71 cm2) and the KM to BP as a substrate was 0.454 mM. Linearity was obtained in concentration range between 0.03 and 0.25 mM BP. The sensitivity was determined to be 649 A/Mxm2 (Figure 4B), which corresponds to (or exceeds) the highest values described in the literature for amperometric analogues (Portaccio et al., 2013; Gayda et al., 2019). For example, the sensor, based on laccase from Trametes vesicolor, immobilized on the surface of graphite electrode, revealed the sensitivity of 232 A/Mxm2 (Gayda et al., 2019). The constructed biosensor has a limit of detection for BP 1.9 µM, close to reported data (Lou et al., 2019), allowing to assay BP level at least in wastewater.
Construction of the laboratory prototype of laccase-based bioreactor for degradation of xenobiotics in model solutions
The development of the effective technologies to restrict the emissions and releases of toxic pollutants, especially, estrogenic chemicals, from the industrial processes and commercial products is an urgent task for environmental scientists. A number of physicochemical methods such as adsorption, membrane-based filtration, Fenton, electrochemical and photochemical degradation, chlorination, ozonation, and ferrate (VI) oxidation has been proposed for the removal of xenobiotics from model aqueous solutions and real wasters (Husain and Qayyum, 2013; Zerva et al., 2019). Unfortunately, these methods have some inherent limitations and therefore cannot be used for large-scale treatment of such pollutants.
The alternative biological methods are looking quite promising and these procedures are helpful in the complete degradation of phenolic compounds. Bacterial, fungal, and algal strains and mixed cultures have successfully been employed for the degradation of hazardous xenobiotics. Recently, the enzymatic methods have attracted the attention of environmentalists for the treatment of endocrine-disrupting compounds. Numerous types of oxidoreductases: laccases, tyrosinases, manganese peroxidase, lignin peroxidase, polyphenol oxidases, horseradish peroxidase, and bitter gourd peroxidase have exhibited their potential for the remediation of such types of compounds (Méndez-Hernández et al., 2015; Wang and Wang, 2016; Rani et al., 2017; Teerapatsakul et al., 2017; Bilal et al., 2019; Fu et al., 2019; Lassouane et al., 2019; Xueping and Shikeren, 2019; Zerva et al., 2019).
A lot of papers reported the application of laccase in bioreactors for utilization/degradation of phenolic compounds. As effective carriers for laccase immobilization, the following matrixes were proposed: glutaraldehyde-modified Ca-alginate beads (Lassouane et al., 2019) and metal-ion-chelated magnetic microspheres (Lin et al., 2016) for BP degradation; copper alginate for Indigo Carmine decolorization (Teerapatsakul et al., 2017); transition metal oxides nanomaterials for degradation of Alizarin Red S dye (Rani et al., 2017); ABTS-modified dual-functionalized cellulose beads for highly improved biodegradation of indole (Xueping and Shikeren, 2019); and magnetic nanoflowers, which possess great efficiency and reusability in the treatment of aqueous solution containing BP (Fu et al., 2019).
In our study, we constructed bioreactors, containing laccase-chitosan beads, and tested their ability to degrade xenobiotics (see Bioreactor construction and investigation section). The model solutions of two xenobiotics (BP and DF) with various concentrations (0.05 and 0.25 mM) were put on the column-type bioreactors. The levels of tested chemicals were monitored by HPLC while running these solutions through bioreactor. Figures 5 and 6 demonstrate the results of BP and DF degradation.
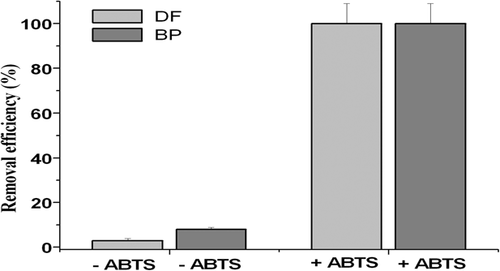
The dynamics of 0.05 mM BP and DF degradation in bioreactors with 0.04 mM laccase were investigated in two variants: with and without ABTS as a mediator of electron transfer. It was demonstrated that the total degradation (100%) of both xenobiotics was achieved during 2 h only in the presence of ABTS, and the efficiencies of DF and BP degradation with mediator were 30- and 12-folds higher, respectively, than in control bioreactors, without ABTS.
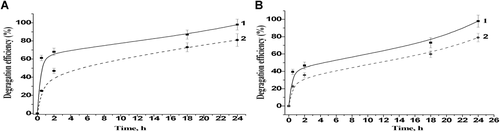
The higher concentrations of BP and DF resulted in the decreased degradation efficiency of laccase-based bioreactors under the described conditions. The abilities of bioreactors with ABTS and different laccase contents (0.04 and 0.4 U/mL) to degrade 0.25 mM xenobiotics were studied, and the results of these experiments are demonstrated in Figure 6. It was shown, that approximately 80 and 98% of both DF and BP destructions were achieved (Figures 6A and 6B, respectively) only after 24 h of incubation in bioreactors with 10-fold higher laccase concentration (0.4 mM).
Conclusions
The screening of fungal strains with a high activity of extracellular laccase was performed. The fungus M. fructicola as the best producer of laccase was chosen and the optimal conditions for the cells cultivation were investigated. Extracellular LA is highest under cultivation at 28°C during 4–5 days in MM with maltose as carbon source, ammonium sulfate as a source of nitrogen, and 0.5 mM Cu2+ ions as an inducer. The simple scheme for laccase isolation and chromatographic purification was proposed, a highly purified enzyme was obtained and its main characteristics were studied. Molecular mass of the laccase subunit was determined to be near 35 kDa, the optimal pH ranges for the highest activity and stability are 4.5–5.0 and 3.0–5.0, respectively, the optimal temperature for LA is 30°C. Laccase preparation was successfully used as a biocatalyst in the ABS and in the bioreactor for bioremediation of phenolic xenobiotics. The ABS based on laccase, immobilized on polyaniline layer, was developed and its analytical characteristics toward BP were studied: sensitivity, 649 A/Mm2; KM, 0.45 mM; and linear range, between 0.03 and 0.25 mM. The laboratory prototype of column-type bioreactor, containing laccase-chitozan beads for degradation of BP and DF was constructed and successfully tested for biodegradation of both xenobiotics in model solutions.
Acknowledgment and funding
The authors are grateful to Mariya Fedorivna Ivash (Institute of Cell Biology NAS of Ukraine) for technical assistance in providing cultivation experiments. This work was financially supported by NAS of Ukraine (Program “Smart Sensory Devices of a New Generation Based on Modern Materials and Technologies”) and by the Ministry of Education and Science of Ukraine (projects 0116U004737 and 0119U100671), grant FEMS-RG-2016-0097.
Author contribution
M.V.G., G.Z.G., and O.M.D. conceived the study, planned the experiments, and analyzed the data. O.M.D., G.Z.G., and D.B. provided the methodology and contributed to the investigation process. O.M.D. and G.Z.G. wrote the original draft of the manuscript. M.V.G. supervised the work. All authors have read and approved the final manuscript.
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.




