lncRNA HCG11 regulates cell progression by targeting miR-543 and regulating AKT/mTOR pathway in prostate cancer
Abstract
Prostate cancer (PCa) is a common cancer worldwide, which mostly occurs in males over the age of 50. Accumulating evidence have determined that long non-coding RNA/microRNA (lncRNA/miRNA) axis plays a critical role in cell progression of cancers, including PCa. However, the pathogenesis of PCa has not been fully indicated. In this study, quantitative real-time polymerase chain reaction was used to detect the expression of HCG11 and miR-543. Western blot was applied to measure the protein expression of proliferating cell nuclear antigen, cleavage-caspase 3 (cle-caspase 3), N-cadherin, E-cadherin, GAPDH, P-AKT, AKT, p-mTOR, and mTOR. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), transwell invasion, and transwell migration assay were used to detect cell proliferation, invasion, and migration, respectively. The function and mechanism of lncRNA HCG11 were confirmed in PCa cell and xenograft mice models. Luciferase assay indicated that miR-543 was a target miRNA of HCG11. Further investigation revealed that overexpression of HCG11 inhibited cell proliferation, invasion, and migration, whereas induced cell apoptosis by regulating miR-543 expression in vitro and in vivo. More than that, lncRNA HCG11 inhibited phosphoinositide-3 kinase/protein kinaseB (PI3K/AKT) signaling pathway to suppress PCa progression. Our data showed the overexpression of HGC11-inhibited PI3K/AKT signaling pathway by downregulating miR-543 expression, resulting in the suppression of cell growth in PCa. This finding proved a new regulatory network in PCa and provided a novel therapeutic target of PCa.
Abbreviation
-
- cle-caspase 3
-
- cleavage-caspase 3
-
- PCa
-
- prostate cancer
Introduction
Prostate cancer (PCa) is the second common cancer and the fifth leading cause of death in males worldwide, which may spread from the prostate to other parts of the body, especially bones and lymph nodes (Nelson et al., 2003; Stewart and Wild, 2014; Siegel et al., 2015). PCa has a high incidence and poor prognosis in males over the age of 50 and most patients die from metastatic PCa (Stewart and Wild, 2014). However, the pathogenesis of PCa remains fully unclear.
Previous studies have reported that non-coding RNAs (ncRNAs) play important roles in cancer development, tumor formation, immune response, and inflammation of many cancers, which includes small ncRNAs (<200 nucleotides at length) and long ncRNAs (lncRNA, >200 nucleotides at length) (Bushati and Cohen, 2007; Krol et al., 2010; Perkel, 2013; Rapicavoli et al., 2013; Tao et al., 2017). Emerging evidence suggested that lncRNA is involved in cell proliferation, invasion, migration, and apoptosis in multiple types of cancers, including PCa (Guo et al., 2016; Li et al., 2016b; Zhang et al., 2017; Lingadahalli et al., 2018). Chakravarty et al. (2014) reported that lncRNA NEAT1 was a critical modulator in cell progression of PCa. More than that, the expression of p21 and PCGEM1 may help to diagnose PCa (Xue et al., 2013; Işın et al., 2015).
lncRNA HCG11 has been demonstrated to participate in the regulation of cell progression and prognosis in hepatocellular carcinoma and squamous carcinoma (Xu et al., 2017; Chen et al., 2018). In addition, downregulation of lncRNA HCG11 in PCa is associated with poor prognosis (Zhang et al., 2016). However, the detailed function and regulatory mechanism of lncRNA HCG11 in cell progression of PCa have not been fully illustrated.
In our study, we found that lncRNA HCG11 was significantly downregulated in PCa tissues and cells. Moreover, lncRNA HCG11 was obviously associated with metastasis tissues compared with non-metastasis tissues in PCa. Overexpression of HCG11 inhibited cell invasion and migration. Meanwhile, the results of bioinformatic analysis predicted that miR-543 was a potential miRNA of lncRNA HCG. Therefore, we hypothesized that lncRNA HGG11 was involved in the cellular progression of PCa by targeting miR-543.
Materials and methods
Patients and tissue specimens
This experiment was approved by the Research Ethics committee of the Fourth Hospital of Hebei Medical University. Twenty pairs of PCa tissues (non-metastasis and metastasis tissues) and adjacent normal tissues were obtained from patients following informed written consent. All patients did not receive any pre-operation treatment.
Cell culture and transfection
Human prostate normal cell lines RWPE1 and human PCa cell lines LNCaP, PC-3, and C4-2B as well as HEK293T cell lines were obtained from RiboBio Co. (Guangzhou, China) and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA), penicillin and streptomycin at 37°C with 5% CO2. Medium was changed every 2 days.
pcDNA3.1-miR-543 (MiR-17-5p mimics, 5′-AAACAUUCGCGGUGCACUUCUU-3′), pcDNA3.1-HCG11(pcDNA-HCG11) and their corresponding negative control (pcDNA3.1-NC, pcDNA3.1-vector) were synthesized and purchased from GenePharma (Shanghai, China). Small interfering RNA (siRNA) (Silencer R; Life Technologies, Shanghai, China) and siRNAs targeting miR-543 (anti-miR-543, 5′-AAGAAGUGCACCGCGAAUGUUU-3′) were constructed by GenePharma. Lipofectamine 2000 (Invitrogen, CA, USA) was applied to transfect all plasmid and oligos according to the manufacturer's instructions.
Quantitative real-time polymerase chain reaction
Total RNAs were extracted from tissues and cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The RNA concentration was measured using spectrophotometry. Complementary DNA (cDNA) of miRNA was reverse transcribed using TaqMan miRNA Reverse-Transcription Kit (Applied Biosystems, Foster City, CA, USA). cDNA of messenger RNA (mRNA) was reverse transcribed using Reverse-Transcription Reagents (Applied Biosystems). Real-time qPCR was performed using SYBRH Select Master Mix for CFX (Invitrogen) following the manufacturer's instructions. The fluorescence was detected in an ABI 7300 System (Applied Biosystems). lncRNA and mRNA expression were normalized according to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). miRNA expression was normalized according to the expression of U6. 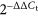 method was used to calculate the relative expression of miRNA and mRNA. The primer sequences: lncRNA HCG11, forward, 5′-GCTCTATGCCATCCTGCTT-3′ and reverse, 5′-TCCCATCTCCATCAACCC-3′.
method was used to calculate the relative expression of miRNA and mRNA. The primer sequences: lncRNA HCG11, forward, 5′-GCTCTATGCCATCCTGCTT-3′ and reverse, 5′-TCCCATCTCCATCAACCC-3′.
GAPDH, forward, 5′-TGTTCGTCATGGGTGTGAAC-3′ and reverse, 5′-ATGGCATGGACTGTGGTCAT-3′.
miR-543, forward, 5′-CCAGCTACACTGGGCAGCAGCAATTCATGTTT-3′ and reverse 5′-CTCAACTGGTGTCGTGGA-3′.
U6, forward 5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-AACGCTTCACGAATTTGCGT-3′.
Western blot
Total protein was extracted from cells lysed in radioimmunoprecipitation assay buffer (Thermo Fisher Scientific, San Jose, CA, USA). Protein concentration was detected using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). Then, the equivalent weights of protein samples (30 µg) were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane. Then, the membrane was blocked in Tris-buffered saline (TBS) with 5% no-fat milk. Subsequently, the membrane was incubated with primary antibody against proliferating cell nuclear antigen (PCNA), cleavage-caspase 3 (cle-caspase 3), N-cadherin, E-cadherin, phosphorylation-AKT (p-AKT), AKT, p-mammalian target of rapamycin (mTOR), mTOR, and GAPDH (1:2,000; Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight. After being washed with TBST buffer, the membrane was incubated with secondary antibodies goat anti-mouse immunoglobulin G (1:2,000 dilution; Cell Signaling Technology) at 37°C for 1 h. The western blot was detected using ECL detection kit (Thermo Fisher Scientific, Rockford, IL, USA), optical densities were measured using ChemiDoc XRS System (Bio-Rad).
Cell proliferation and apoptosis
Cell proliferation was detected using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded into 96-well plates at a density of 2 × 103 cells per well after transfection. Cultured 48 h later, 20 µL MTT solution (5 mg/mL, Sigma-Aldrich, St. Louis, MO, USA) was added into each well to stain all cells and the plate was incubated 37°C for 4 h. Then 200 µL of dimethyl sulfoxide (DMSO; Sigma-Aldrich) were added into each well. Cell proliferation was performed by detecting the absorbance at 490 nm using a Multiskan Ascent 354 microplate reader (Thermo Labsystems, Waltham, MA, USA).
Cell apoptosis was measured using flow cytometry. Cells were trypsinized and washed with phosphate-buffered saline. Then, each sample was stained with FITC-Annexin V and PI (BD Biosciences, San Jose, CA, USA) for 30 min. Cell apoptosis was determined by flow cytometry (BD Biosciences).
Cell invasion and migration
Cell invasion was performed using Transwell chamber with Matrigel (BD Biosciences) and cell migration was displayed using Transwell chamber without Matrigel. 1 × 105 cells were seeded into the upper chamber and DMEM with 10% FBS was added into the lower chamber. After the plate was incubated at 37°C for 24 h, the cells on the upper chamber were wiped away. Then, the membrane was fixed with 90% ethyl alcohol at 37°C for 10 min and was stained with 0.2% crystal violet for 30 min. The cell migration and invasion were counted using an inverted microscope.
Dual-luciferase reporter assay
3′-Untranslated region (3′-UTR) of lncRNA HCG11 was amplified using PCR and inserted into pMIR-REPORT™ (Thermo Fisher Scientific) to construct XISY wild-type reporter vector (HCG11-wt). The HCG11 mutant-type (HCG11-mut) was made with GeneArt™ Site-Directed Mutagenesis PLUS System (Thermo Fisher Scientific). Then, HCG11-WT or HCG11-MUT and miR-NC or miR-543 were transfected into HEK293T cells. Otherwise, HCG11-wt or HCG11-mut and anti-NC or anti-miR-543 were transfected into HEK293T cells. After transfection for 48 h, the luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) following the protocols. The Renilla luciferase activity was normalized to calculate firefly luciferase activity.
Animal experiment
HCG11 and negative control (vector) were stably transfected into C4-2B cell lines. Then the transfected cell lines were injected subcutaneously to the right side of the posterior flank of female BALB/c nude mice (Beijing Vital River Laboratory Animal Technology Co., Ltd. China) at 4–5-week-old. Tumor volumes were measured every 5 days. After injected for 30 days, all mice were sacrificed. Tumor xenografts were collected and weighted. The animal experiment was approved by the Institutional Animal Care and Use Committee of the Fourth Hospital of Hebei Medical University.
Statistical analysis
The analysis of all data was performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). All comparisons were analyzed using a Student's t test to assess the significance of the results unless otherwise specified. Pearson's correlation analysis was used to analyze the correlation between HCG11 expression and miR-543 expression. P < 0.05 was considered to be statistically significant.
Results
LncRNA HCG11 was downregulated in PCa tissues and cells
To confirm the expression of HCG11 in PCa tissues and cells, qRT-PCR assay was used in this experiment. As shown in Figure 1A, HCG11 has lower expression in non-metastasis and metastasis tissues compared with that in normal tissues. This result indicated that lncRNA HCG11 was downregulated in PCa tissues and was associated with metastasis in PCa. Then, we obtained the normal cell line (RWPE1) and PCa cell lines (LNCaP, PC-3, and C4-2B) for the following experiments, the results showed that HCG11 expression was obviously decreased in prostatic carcinoma cells compared with that in normal cell lines (RWPE1) and was lowest in C4-2B cell line (Figure 1B).
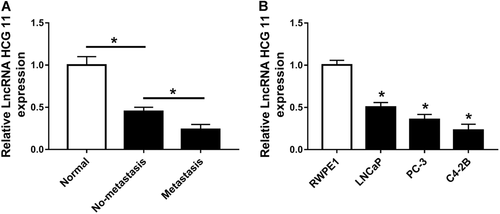
LncRNA HCG11 was downregulated in PCa tissues and cells. (A) qRT-PCR analysis of the relative expression of lncRNA HCG in PCa tissues and normal tissues. (B) qRT-PCR analysis of the relative expression of lncRNA HCG in PCa cell lines (LNCaP, PC-3, and C4-2B) and normal cell line (RWPE1). lncRNA, long non-coding RNA; PCa, prostate cancer; qRT-PCR, quantitative real-time polymerase chain reaction.
Upregulated lncRNA HCG11 expression inhibited cell growth and induced apoptosis in PCa
To further investigate the function of HCG11 in PC, we constructed the cell lines of transfection with HCG11, which significantly increased HCG11 expression (Figure 2A). Then the cell viability of HCG11 was measured using the MTT assay. The result showed that overexpressed HCG11 was significantly lower than that of vector and control cell lines (Figure 2B). Meanwhile, flow cytometry showed that the apoptosis cells with overexpressed HCG11 were sharply increased compared with that of vector and control cells (Figure 2C). Additionally, high HCG11 expression significantly inhibited the PCNA protein level and induced cle-caspase 3 protein expression (Figure 2D).
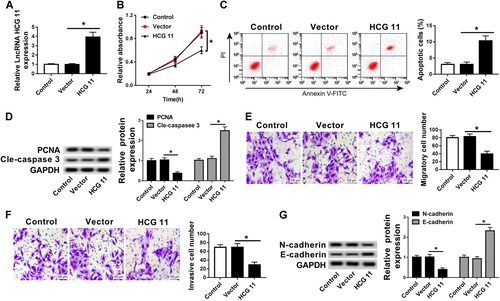
Upregulated lncRNA HCG11 expression inhibited cell growth and induced apoptosis in PCa. (A) qRT-PCR analysis of the relative expression of lncRNA HCG in control, vector, and HCG11 groups. (B) MTT assay analysis of cell proliferation in control, vector, and HCG11 groups. (C) Flow cytometry analysis of cell apoptosis in control, vector, and HCG11 groups. (D) Western blot analysis of the relative expression of PCNA and cle-caspase 3 in control, vector, and HCG11 groups. (E and F) Transwell assay analysis of cell metastasis (E) and invasion (F) in control, vector, and HCG11 groups. (G) Western blot analysis of the relative expression of N-cadherin and E-cadherin in control, vector, and HCG11 groups. cle-caspase 3, cleavage-caspase 3; lncRNA, long non-coding RNA; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PCa, prostate cancer; PCNA, proliferating cell nuclear antigen; qRT-PCR, quantitative real-time polymerase chain reaction.
More than that, we also investigated the effects of HCG11 on tumor metastasis and invasion. The transwell assay displayed that the metastasis and invasion of PC cells lines was decreased by HCG11 overexpression (Figures 2E and 2F). Moreover, the protein level of N-cadherin was decreased and the protein level of E-cadherin was increased by upregulating HCG11 (Figure 2G). Therefore, these results showed that upregulated HCG11 expression could inhibit cell proliferation, metastasis, and invasion as well as induced cell apoptosis in PCa.
miR-543 was a target miRNA of lncRNA HCG11
On the basis of the bioinformatics analysis, we found that miR-543 had complementary sequences with HCG11 3′-UTR (Figure 3A). The results of luciferase reporter assay showed that miR-543 mimic decreased the luciferase activity by binding with HCG11-3′-UTR (wt), while HCG11-mut has no effect with it (Figure 3B). Moreover, anti-miR-543 increased the luciferase activity by binding with HCG11-3′-UTR (wt) rather than HCG11-mut (Figure 3C). In addition, overexpression of HCG11 greatly decreased the expression of miR-543, whereas downregulation of HCG11 remarkably increased the miR-543 expression (Figure 3D).
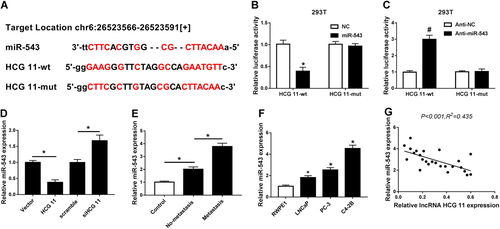
miR-543 was a target miRNA of lncRNA HCG11. (A) The bioinformatics analysis was applied to predict the binding sites between miR-543 and HCG11. (B and C) Luciferase activity was detected using luciferase reporter assay. (E) qRT-PCR analysis of the relative expression of miR-543 in PCa tissues and normal tissues. (D) qRT-PCR analysis of the relative expression of miR-543 in vector, HCG11, scramble, and siHCG11 groups.(F) qRT-PCR analysis of the relative expression of miR-543 in PCa cell lines (LNCaP, PC-3, and C4-2B) and normal cell line (RWPE1). (G) Analysis of correlation between HCG11 and miR-543 expression in PCa tissues. lncRNA, long non-coding RNA; miRNA, microRNA; PCa, prostate cancer; qRT-PCR, quantitative real-time polymerase chain reaction.
Then, we further investigated the association between HCG11 and miR-543 using qRT-PCR. As shown in Figures 3E and 3F, miR-543 was upregulated in PCa tissues and cells compared with normal tissues and cells (Figures 3E and 3F). Moreover, there was a negative correlation between HCG11 and miR-543 expression in PC tissues (Figure 3G). These results showed that the suppressive effect of HCG11 occurred via inhibiting miR-543 expression.
miR-543 was involved in HCG11-mediated regulation of cell proliferation, apoptosis, migration, and invasion in PCa cells
Next, we investigated the underlying mechanism of HCG11 in PCa cells and validated the function of miR-543 in HCG11-mediated PCa process. The control, vector and HCG11 were transfected into PCa cells, respectively. Meanwhile, HCG11 was co-transfected with miR-543 or miR-NC into PCa cells, respectively. HCG11 transfection significantly decreased the miR-543 expression (Figure 4A). In addition, compared with HCG11 + miR-NC group, the expression of miR-543 was obviously increased in HCG11 + miR-543 group (Figure 4A). Thus, miR-543 transfection improved miR-543 expression. According to the results of MTT assay and flow cytometry, we found that the overexpression of HCG11 inhibited cell proliferation and induced cell apoptosis in PCa cells, while the overexpression of miR-543 significantly blocked the effects of high HCG11 expression on PCa cell proliferation and apoptosis (Figures 4B and 4C). Meanwhile, Figure 4D revealed that PCNA protein expression was inhibited but cle-caspase 3 protein expression was induced by overexpression of HCG11, while these effects were impaired by upregulating miR-543.
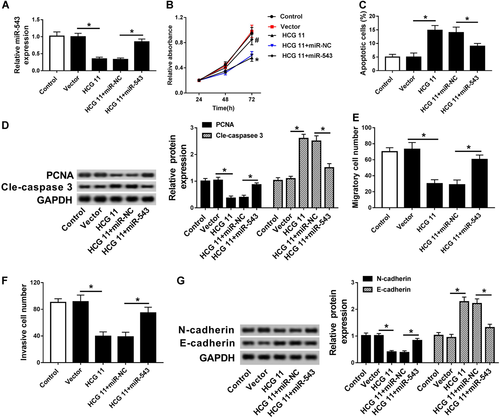
miR-543 was involved in HCG11-mediated regulation of cell proliferation, apoptosis, migration, and invasion in PCa cells. (A) qRT-PCR analysis of the relative expression of miR-543 in control, vector, HCG11, HCG11 + miR-NC, and HCG11 + miR-543 groups. (B) MTT assay analysis of cell proliferation in control, vector, HCG11, HCG11 + miR-NC, and HCG11 + miR-543 groups. (C) Flow cytometry analysis of cell apoptosis in control, vector, HCG11, HCG11 + miR-NC, and HCG11 + miR-543 groups. (D) Western blot analysis of the relative expression of PCNA and cle-caspase 3 in control, vector, HCG11, HCG11 + miR-NC, and HCG11 + miR-543 groups. (E and F) Transwell assay analysis of cell metastasis (E) and invasion (F) in control, vector, HCG11, HCG11 + miR-NC and HCG11 + miR-543 groups. (G) Western blot analysis of the relative expression of N-cadherin and E-cadherin in control, vector, HCG11, HCG11 + miR-NC, and HCG11 + miR-543 groups. cle-caspase 3, cleavage-caspase 3; miR, microRNA; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PCNA, proliferating cell nuclear antigen; qRT-PCR, quantitative real-time polymerase chain reaction.
Furthermore, transwell migration and invasion analyses performed that HCG11 transfection significantly decreased PCa cell invasion and migration, which were restored by miR-543 overexpression (Figures 4E and 4F). More than that, overexpression of HCG11 inhibited N-cadherin protein expression, whereas enhanced E-cadherin protein expression, upregulation of miR-543 reversed the effects of HCG11 transfection on the protein level of N-cadherin and E-cadherin (Figure 4G). These findings demonstrated that miR-543 was a functional target miRNA of HCG11 and that upregulation of miR-543 could reverse the effects of high HCG11 expression on PCa cell proliferation, apoptosis, invasion, and migration.
LncRNA HCG11 inhibited phosphoinositide-3 kinase/protein kinase B (PI3K/AKT) pathway to suppress PCa progression
PI3K/AKT pathway is an important signaling pathway in the regulation of cell cycle, which is associated with oncogenesis and development (Skeen et al., 2006; Iżycka-Świeszewska et al., 2010). P-AKT, AKT, p-mTOR, and mTOR were crucial proteins of PI3K/AKT pathway, which belonged to a part of the PI3K/PKB/AKT/mTOR pathway. P-AKT and p-mTOR protein expressions were significantly inhibited or induced, while AKT and mTOR protein expressions showed no significant changes, suggesting that PI3K/AKT pathway was inhibited or upregulated, so we measured their protein levels with western blot. The results showed that upregulation of HCG11 significantly reduced p-AKT and P-mTOR protein expression, whereas overexpression of miR-543 largely reversed the effects of upregulation of HCG11 on P-AKT and p-mTOR protein expression in PCa cells. However, AKT and mTOR protein expression has no significant change in all groups (control, vector, HCG11, HCG11 + miR-NC, and HCG11 + miR-543 groups) (Figure 5). These findings showed that lncRNA HCG11 affected PCa cell progression by inhibiting PI3K/AKT pathway.
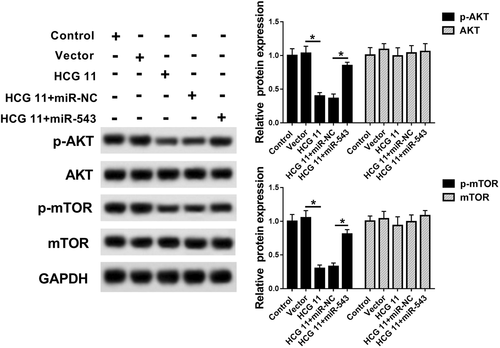
lncRNA HCG11 inhibited PI3K/AKT pathway to suppress PCa progression. Western blot analysis of the relative protein expression of p-AKT, AKT, p-mTOR, and mTOR in control, vector, HCG11, HCG11 + miR-NC, and HCG11 + miR-543 groups. lncRNA, long non-coding RNA; mTOR, mammalian target of rapamycin; PCa, PI3K/AKT, prostate cancer; phosphoinositide-3 kinase/protein kinase B.
LncRNA HCG11 regulated tumor growth by inhibiting miR-543 in vivo
To further verify the role of lncRNA HCG11 in tumor growth in vivo, C4-2B cells transfected with vector or HCG11 were injected into the flank of nude mice, respectively. As shown in Figure 6A, after injection for 25 days to 30 days, tumor volume of HCG11 was significantly lower than that of vector. The mice underwent surgery and the tumor weight was measured after 30 days of injection, tumor weight was notably reduced in HCG11 group compared with that in vector group (Figure 6A). Additionally, HCG11 transfection improved HCG11 expression but reduced miR-543 expression (Figures 6B and 6C). These findings demonstrated that HCG11 inhibited tumor growth via downregulating miR-543 in PCa.
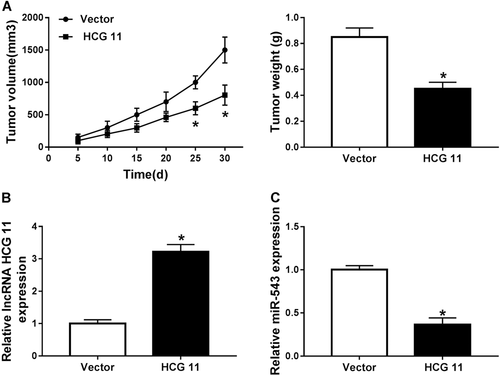
lncRNA HCG11 regulated tumor growth by inhibiting miR-543 in vivo. (A) Effects of lncRNA HCG11 on tumor growth in vivo. (B and C) qRT-PCR analysis of the relative expression of lncRNA HCG11 (B) and miR-543 (C) in vector and HCG groups in vivo. lncRNA, long non-coding RNA; miR, microRNA; qRT-PCR, quantitative real-time polymerase chain reaction.
Discussion
With the development of biotechnology, the molecular mechanism of PCa has been determined. Multiple lncRNAs, as tumor suppressors or oncogenes, have been shown to be involved in PCa processes, including cancer development, growth, and metastasis. In gastric cancer, lncRNA H19 was upregulated and contributed to carcinogenesis and metastasis (Yang et al., 2012). In addition, lncRNA GAS5 was downregulated in cervical cancer and knockdown of lncRNA GAS5 promoted cell growth and predicted a poor prognosis (Cao et al., 2014). In our study, lncRNA HCG11 expression was significantly reduced in PCa tissues and cells in accordance with a study by Zhang et al. (2016). Thus, we obtained the cell lines that overexpressed lncRNA HCG11 to verify its function in PCa cells. The results demonstrated that overexpressed lncRNA HCG11 significantly decreased cell proliferation, invasion and migration, while induced cell apoptosis. Otherwise, the protein level of PCNA and caspase 3 were related to cell proliferation and apoptosis, respectively. N-cadherin and E-cadherin were key proteins of EMT, which regulated cell metastasis. The change of these proteins further verified the regulatory function of overexpression of HCG11 in PCa. These findings suggested that overexpressed HCG11 inhibited the regulation of cell growth in PCa.
Given the significant effects of lncRNA HCG11 on cancer proliferation, apoptosis, invasion, and migration in PCa, as determined by our experiments, the regulatory mechanism of lncRNA HCG11 urgently need to be investigated. It is known that lncRNA usually acts as a ceRNA to bind to miRNA, which regulates cellular processes in cancers, including PCa. For example, lncRNA H19 repressed PCa metastasis by regulating miR-675 (Zhu et al., 2014). lncRNA GAS5/miR-103 axis, lncRNA SNHG1/miR-199-3p axis, and lncRNA XIST/miR-23a axis were involved in PCa cell proliferation, metastasis, and apoptosis (Dong et al., 2016; Du et al., 2017b; Li et al., 2017). In this study, we validated that miR-543 was a target miRNA of HCG11 using luciferase reporter assay for the first time.
Increasing evidence reported that miR-543 promoted cell growth in many cancers, such as gastric cancer, ovarian cancer, and PCa (Du et al., 2017a; Li et al., 2016a; Song et al., 2015). Du et al. (2017a) suggested that miR-543 was upregulated in metastasis tissues in prostate and increased the cell proliferation and metastasis. In our study, miR-543 expression was overexpressed in PCa tissues and cells. Moreover, the expression of HCG11 was negatively correlated with miR-543 expression in PCa cells.
According to our study, to clarify the underlying regulatory mechanism of HCG11 and miR-543 in PCa, HCG11 and miR-543 were co-transfected into C4-2B cells. We found that upregulated miR-543 reversed the effect of high HCG11 expression on PCa cells. More than that, this regulatory mechanism was verified in mice model, the results suggested that upregulation of HCG11 could inhibit tumor growth via downregulation of miR-543.
AKT/mTOR signaling pathway is a critical signaling pathway in cellular process of cancers, which is closely related to cell apoptosis, migration, invasion, and differentiation (Gao et al., 2003, 2004; Peltier et al., 2007). Besides, we also found that miR-543 was closely associated with PI3K/AKT pathway. Studies have shown that miR-543 targets KLF4, which regulates cell proliferation via regulation of PI3K/AKT signaling in cancers (Zhai et al., 2017; Tang et al., 2018). In the present study, upregulation of HCG11 inhibited AKT/mTOR signaling pathway, whereas upregulated miR-543 activated the AKT/mTOR signaling pathway inhibited by the overexpression of HCG11.
In conclusion, we identified that miR-543 was a target miRNA of HCG11. Otherwise, we found that HCG11 played an important role in PCa cell progression and tumor growth by regulating AKT/mTOR signaling pathway by modulating miR-543 expression, thereby further improving the understanding of regulatory mechanism in PCa.
Acknowledgment
We kindly acknowledge the Fourth Hospital of Hebei Medical University who hosted the research.
Conflict of interest
The authors declare that there are no conflict of interest.




