The influence of serum-supplemented culture media in a transwell migration assay
Siti Sarah Omar Zaki and Livashini Kanesan contributed equally to this work.
Abstract
Our work cautions against the use of serum-supplemented culture media in a transwell migration assay when using chemoattractants other than FBS. At 24 h, a 5% foetal bovine serum (FBS) gradient caused BV2 microglia to migrate toward the lower compartment of the transwell apparatus. Interestingly, FBS-supplemented media in the absence of a gradient also resulted in notable microglia migration. Serum can therefore confound the interpretation of a transwell migration assay when another chemoattractant is used.
Abbreviations
-
- ATP
-
- adenosine triphosphate
-
- CNS
-
- central nervous system
-
- FBS
-
- foetal bovine serum
-
- LPS
-
- lipopolysaccharide
Introduction
Cell migration occurs during embryogenesis, inflammation, wound healing, and in cancer metastasis (Lauffenburger and Horwitz, 1996). In inflammation, leucocytes migrate toward the area of tissue damage to initiate immune responses such as phagocytosis. There are several techniques for evaluating migration in vitro including the Boyden chamber or transwell migration assay, Dunn chamber assay, and the wound healing assay (also known as the scratch assay or gap closure assay).
The transwell migration assay consists of a culture plate insert with a porous membrane lining. Cells are seeded in the upper compartment of the insert and cells migrate through the membrane to the lower compartment of the culture plate that contains a chemotactic stimulus (Wu et al., 2014). Serum is often utilised in transwell migration protocols to provide chemotactic stimuli. For this, a chemoattractant gradient is created by placing cells in the transwell insert with serum-free media and adding serum to the lower chamber (Li et al., 2011; Zhu et al., 2016). It is unclear however, what effect serum has on cell migration when used as a supplement in cell culture media.
Transwell migration assays are often used to determine in vitro migratory abilities of microglia (Miller and Stella, 2009; Zhang et al., 2014; Maeda et al., 2016). Microglia are tissue-specific macrophages of the central nervous system (CNS) (Ransohoff and Cardona, 2010). They have highly motile cellular processes that continuously sample for homoeostatic changes in the brain parenchyma (Nimmerjahn et al., 2005). When there is stress or tissue damage, microglia respond by migrating rapidly toward the area of injury and mediating inflammatory responses (Davalos et al., 2005). Migration therefore serves as an indicator of microglia activation.
We determined the effect of setting up a transwell migration assay for BV2 microglia with 5% foetal bovine serum (FBS)-supplemented culture media. BV2 microglia are an immortalised cell line that share many phenotypic characteristics of microglia isolated from brain tissue (Blasi et al., 1990). FBS at a concentration of 5% is used to maintain BV2 microglia cultures for propagation and also certain downstream assays (Rahmat et al., 2013; Jose et al., 2014). It is important to consider the effect of using serum-supplemented media in a transwell migration assay when other chemoattractants such as lipopolysaccharide (LPS) or cell-conditioned media are used (Ren et al., 2008; Rahmat et al., 2013).
Materials and methods
BV2 microglia culture
BV2 microglia were a generous gift from Professor Thameem Dheen, National University Singapore. They are an immortalised murine microglia cell line infected with a v-raf/v-myc oncogene carrying retrovirus J2 (Blasi et al., 1990). The cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% (v/v) heat-inactivated FBS, 1% (v/v) penicillin–streptomycin, 1% (v/v) gentamycin, 0.5% (v/v) fungizone, 1% (v/v) 100× non-essential amino acids (all Gibco, USA) and 0.1% (v/v) insulin (Sigma-Aldrich, St. Louis, MO, USA).
Transwell migration assay
BV2 (1 × 105 cells) were seeded onto transwell inserts with a polyethylene terephthalate membrane pore size of 8 μm (Thermo Fisher Scientific) in 24-well plates with the various 5% FBS configurations as depicted in Figure 1. After 24 h, media within the transwell inserts were carefully removed. Cells were fixed with 2% paraformaldehyde, permeabilised with 0.01% Triton X-100 (Sigma-Aldrich), and stained with crystal violet (Sigma-Aldrich). Cells that did not migrate across the transwell membrane were then removed by gently wiping with a cotton swab. Migrated cells were viewed with a phase-contrast microscope (Olympus BX51-FL-CCD), imaged with an Olympus XC50 camera using the anaLYSIS software and processed using ImageJ software.
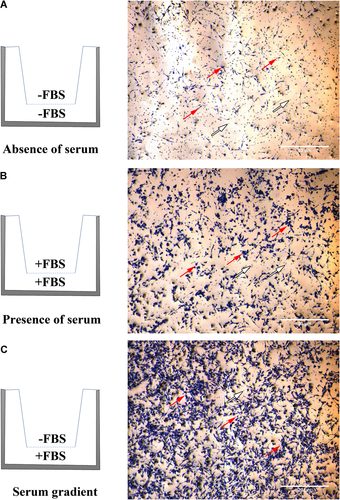
The effect of serum on BV2 microglia migration. BV2 microglia (1.0 × 105 cells/well) were seeded onto 8 μm transwell inserts in 24-well plates in the absence (A) or presence (B) of 5% foetal bovine serum (FBS) or an FBS gradient (C). Migration was assessed at 24 h by staining BV2 cells with crystal violet and viewing them under a phase-contrast microscope. Red arrows depict migrated BV2 microglia cells and white arrows depict the pores of the transwell membrane. Images are representative of three independent experiments.
Results
At 24 h, a minimal amount of BV2 migration occurred in the absence of serum (Figure 1A; −FBS/−FBS). Distinct migration occurred in the presence of a serum gradient (Figure 1C; −FBS/+FBS), but BV2 migration also occurred in wells without a gradient (Figure 1B; i.e., FBS present in both the transwell insert and lower compartment; +FBS/+FBS). When compared with BV2 migration performed in the absence of serum, the number of microglia that migrated in the presence of serum is higher. Therefore, BV2 microglia cultured in serum-free media is necessary for transwell migratory assays as they demonstrate minimal migration in the absence of chemoattractive stimuli. When seeded in the absence of serum, BV2 microglia do migrate noticeably towards an adenosine triphosphate stimulus (Supplementary Figure 1).
Discussion
FBS is often used as a chemoattractant in transwell migration assays, typically at a concentration ranging from 2 to 10% in the lower chamber with serum-free media in the transwell insert to form a gradient (Li et al., 2011; Zhu et al., 2016; Zhen et al., 2017). However for assays that utilise chemoattractants other than serum, the use of serum-free media may be less stringent (Ren et al., 2008; Rahmat et al., 2013; Li et al., 2017). Serum is commonly supplemented in culture media as it is a vital source of growth and adhesion factors, hormones and lipids for cells.
We determined the effect of setting up a transwell migration assay for BV2 microglia with the presence of 5% FBS in the culture media. This is the concentration of FBS used to maintain BV2 cells in culture in our laboratory (Rahmat et al., 2013; Jose et al., 2014). The BV2 microglia cell line is a murine-origin primary microglia culture that has been immortalised with the v-raf/v-myc oncogene. They retain many of the phenotypic aspects of primary microglia including phagocytic activities, inflammatory cytokine expression and are positive for microglia markers including Mac1 and CD40 (Blasi et al., 1990). Remarkably, the presence of serum in the culture media induced notable migration of BV2 cells after 24 h, even in the absence of a serum gradient. This may be due to several reasons including (i) a serum gradient that forms naturally within the well over the 24-h period, and (ii) serum within the transwell insert and lower compartment stimulate BV2 cells to proliferate and the cells migrate to the converse side of the insert membrane due to a contest for space. The latter is supported by the observation that the cell density in the transwell insert that contains serum appear higher compared to cells in transwell inserts without serum (Supplementary Figure 2).
To conclude, culture media supplemented with serum provide a migratory stimulus for BV2 microglia even in the absence of a serum gradient. Along with the variation between different batches of serum, this can affect the readout of migration assays due to the different proportion of soluble factors present. Therefore, we recommend that transwell migration assays to be performed with serum-free culture media when utilising chemoattractants other than serum.
Acknowledgements and funding
The authors thank Dr. Manraj Singh Cheema, Shamin Azwar, Thiban Sandramurthi, Tong Chih Kong and Tan Shi Wei for discussions on the experiments. This study was funded by the Geran Putra–Inisiatif Putra Siswazah (Universiti Putra Malaysia) [GP-IPS7]. Siti Sarah Omar Zaki is supported by the Graduate Research Fellowship UPM and recipient of the Gift Her With Life Education Grant.
Author contribution
S.S.O.Z. and L.K. designed and performed the experiments. S.V. conceived and designed the study. S.V. wrote the manuscript. S.S.O.Z., L.K., and S.V. analysed the data. All authors interpreted the data and read and approved the final manuscript.




