A novel role for integrin-linked kinase in periodic mechanical stress-mediated ERK1/2 mitogenic signaling in rat chondrocytes
Abstract
In recent years, a variety of studies have been performed to investigate the cellular responses of periodic mechanical stress on chondrocytes. Integrin β1-mediated ERK1/2 activation was proven to be indispensable in periodic mechanical stress-induced chondrocyte proliferation and matrix synthesis. However, other signal proteins responsible for the mitogenesis of chondrocytes under periodic mechanical stress remain incompletely understood. In the current investigation, we probed the roles of integrin-linked kinase (ILK) signaling in periodic mechanical stress-induced chondrocyte proliferation and matrix synthesis. We found that upon periodic mechanical stress induction, ILK activity increased significantly. Depletion of ILK with targeted shRNA strongly inhibited periodic mechanical stress-induced chondrocyte proliferation and matrix synthesis. In addition, pretreatment with a blocking antibody against integrin β1 resulted in a remarkable decrease in ILK activity in cells exposed to periodic mechanical stress. Furthermore, inhibition of ILK with its target shRNA significantly suppressed ERK1/2 activation in relation to periodic mechanical stress. Based on the above results, we identified ILK as a crucial regulator involved in the integrin β1-ERK1/2 signal cascade responsible for periodic mechanical stress-induced chondrocyte proliferation and matrix synthesis.
Abbreviations
-
- DMSO
-
- dimethyl sulfoxide
-
- ECM
-
- extracellular matrix
-
- ERK
-
- extracellular signal-regulated kinase
-
- GAG
-
- glycosaminoglycan
-
- GAPDH
-
- glyceraldehyde-3-phosphate dehydrogenase
-
- HRP
-
- horseradish peroxidase
-
- ILK
-
- integrin-linked kinase
-
- Lv
-
- lentivirus vector
-
- NT
-
- non-targeting
Introduction
Periodic mechanical stress has been demonstrated to promote chondrocyte proliferation and matrix synthesis and thus improve the quality of tissue-engineered cartilage, but the detailed mechanisms were not completely understood (Ren et al., 2011; Ren et al., 2012; Liang et al., 2013; Liang et al., 2015). Identifying the underlying molecular mechanisms by which chondrocytes sense and respond to periodic mechanical stress would provide convincing methods for constructing better quality tissue-engineered cartilage in future. Mechanoreceptors and the associated signaling molecules have been demonstrated to play indispensable roles in mechanotransduction pathways in chondrocytes (Bougault et al., 2013; Ozawa et al., 2015). Integrin is the core mechanoreceptor that mediates mechanically induced cell behavior and function (Raizman et al., 2010; Bougault et al., 2013). Our previous results revealed that periodic mechanical stress could promote chondrocyte proliferation and matrix synthesis through the integrin β1-ERK1/2 signal cascade, but other signaling proteins participating in the signal cascade remain to be explored (Ren et al., 2011; Ren et al., 2012; Liang et al., 2013).
Integrin-linked kinase (ILK), an intracellular serine-threonine kinase, was identified as a critical component which binds to the cytoplasmic domains of the β-integrin subunits and plays a key role in integrin-associated signal transduction (Ma et al., 2016; Troyano et al., 2015). Based on the finding that integrin β1-mediated periodic mechanical stress induced mitogenic effects in chondrocytes, and repetitive deformation could activate ILK signaling in intestinal epithelial cells, we hypothesized that ILK may be activated by exposure to periodic mechanical stress in chondrocytes (Yuan et al., 2011; Ren et al., 2012). Increasing evidence suggested a pivotal role of ILK in several non-chondrocytic cell types where it affects cellular responses including protein synthesis and migration under cyclic strain (Maier et al., 2008; Yuan et al., 2011). For example, the work of Maier et al. (2008) showed that cyclic strain-stimulated tenascin-C expression was significantly reduced upon ILK inhibition. Furthermore, ILK was also confirmed to participate in the regulation of cellular biological effects in chondrocytes under several non-mechanical stimuli (Grashoff et al., 2003; Terpstra et al., 2003). However, the detailed functions of ILK in chondrocytes subjected to periodic mechanical stress remain enigmatic. Thus, in the context of periodic mechanical stress, the activation of ILK and the effects of ILK on chondrocyte proliferation and matrix synthesis were investigated.
Our earlier study identified that ERK1/2 was the downstream mediator responsible for integrin β1-initiated chondrocyte proliferation and matrix synthesis under conditions of periodic mechanical stress (Ren et al., 2012). ILK activity has been extensively implicated in activation of the PKB/AKT and GSK-3β pathway (Piao et al., 2014; Yoon et al., 2016). Several researchers also provided evidence for a crucial role of ILK in the phosphorylation of ERK1/2 in some non-chondrocytic cell types (Su et al., 2010; Wang et al., 2014). However, the relationship between ILK and activation of ERK1/2 in chondrocytes exposed to periodic mechanical stress was unknown. In addition, despite a diverse array of studies demonstrating the obvious importance of ILK in integrin-associated signaling, no follow-up studies were performed to probe the precise relationships between ILK and integrin β1 in chondrocytes under periodic mechanical stress (Morris et al., 2015; Smeeton et al., 2016). Thus, the upstream and downstream relationships between integrin β1, ERK1/2, and ILK in chondrocytes under conditions of periodic mechanical stress needed to be explored.
To address these issues, the main aim of the present study was to investigate whether ILK signaling was activated, and then required for cellular processes in chondrocytes under periodic mechanical stress. Additionally, we characterized the association of ILK with integrin β1 and ERK1/2 to link these signals into the mitogenic cascades.
Materials and methods
Animals, chemicals, and strain
Two-week-old Sprague–Dawley (SD) rats of both sexes were provided by the Animal Center of Nanjing Medical University. Fetal bovine serum was purchased from Hangzhou Sijiqing Biological Engineering Materials Co., Ltd. (Zhejiang, China). DMEM-F12, trypsin, collagenase II, and type II collagen were obtained from Sigma (St. Louis, MO, USA). Cell Counting Kit-8 (CCK-8) was acquired from Beyotime Institute of Biotechnology (Jiangsu, China). Protein A/G beads were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Blocking antibodies against integrin β1 were supplied by BD Biosciences (Franklin Lakes, NJ, USA). Anti-GSK3β antibody, anti-phospho-GSK3β rabbit antibody, anti-ERK1/2 rabbit antibody, anti-phospho-ERK1/2 (Thr202/Tyr204) rabbit antibody, and HRP-goat anti-rabbit IgG were supplied by Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-ILK rabbit antibody was supplied by Abcam, (Cambridge, MA, USA). HRP-conjugated GAPDH antibody was supplied by Kang Chen (Shanghai, China, KC-5G5). ECL was purchased from Thermo Scientific (Waltham, MA, USA). RNAiso Plus, PrimeScript RT Reagent Kit, and SYBR Premix Ex Taq II were acquired from TaKaRa (Shiga, Japan). ILK shRNA lentiviral particles, control shRNA lentiviral particles, and Polybrene were supplied by Genechem (Shanghai, China).
A cell culture incubator (Heraeus BB 5060), an air-tight cell culture device and reciprocating pressure pump, barrier type pressure transducer, and inverted microscope equipped with a camera system were acquired from Heraeus (Hanau, Germany), Taixing Experimental Instrument Factory (Jiangsu, China), Tianjin Plastics Research Institute (Tianjin, China), and Olympus (Tokyo, Japan), respectively.
Cell culture
Chondrocytes were harvested using the method described by Seguin and Bernier (2003). Briefly, under sterile conditions, cartilage derived from the limb joints of 2-week-old SD rats was harvested and sliced into 1 mm3 pieces. Tissues were digested with 0.25% trypsin at 37°C for 30 min, followed by digestion with 0.2% collagenase II at 37°C for 4 h. Cells were harvested by filtering with a 200-mesh filter and cultured in DMEM-F12 medium supplemented with 10% FBS in an incubator at 37°C with 5% CO2. The cell population was purified by repeated adherence, and morphology was examined under an inverted phase-contrast microscope following staining with a collagen type II antibody according to the conventional ABC method. Second-generation cells were seeded onto glass slides (25 mm × 25 mm) coated with type II collagen at a density of 105 cells/slide. Experiments were performed when cells reached 70–80% confluence.
Integrin β1 inhibitor
A blocking antibody against integrin β1 was used as a specific inhibitor of integrin β1. It was dissolved in DMEM to form a 1,000× concentrated solution, and aliquots were stored at −20°C. This concentrated solution was diluted 1000× immediately prior to use. Cells were pre-treated with blocking antibodies against integrin β1 (10 µg/mL) or an equivalent amount of DMEM for 5 h.
Construction of a periodic mechanical stress field
A periodic stress field encompassing the perfusion culture system with adjustable stress intensity and frequency was constructed by connecting a reciprocating intensifier pump to the air-tight cell culture device through a barrier-type pressure transducer, as described previously (Nong et al., 2010). In an earlier study, we showed that rat chondrocytes subjected to stress varying from 0 to 200 kPa at 0.1 Hz yielded tissue-engineered cartilage of the best quality (Yue et al., 2007; Zhang et al., 2007). Accordingly, a pressure range of 0–200 kPa and 0.1 Hz frequency were used in the current study.
ILK assay
ILK enzymatic activity was assessed according to previously reported protocols [0.5% sodium deoxycholate, 1% Nonidet P-40, 50 mM HEPES (pH 7.4), 150 mM NaCl] (Tseng et al., 2010). In brief, after immunoprecipitation of ILK with anti-ILK antibody overnight at 4°C from 250 mg of lysate, we resuspended the beads at 30°C for 30 min in 30 µL of kinase buffer containing 1 µg of recombinant substrate [glycogen synthase kinase 3β (GSK3β) fusion protein] and 200 µmol/L cold ATP. Total GSK3β protein and the phosphorylated substrate were detected by Western blot analysis with GSK3β and phospho-GSK3β antibodies, respectively.
Transfection analysis
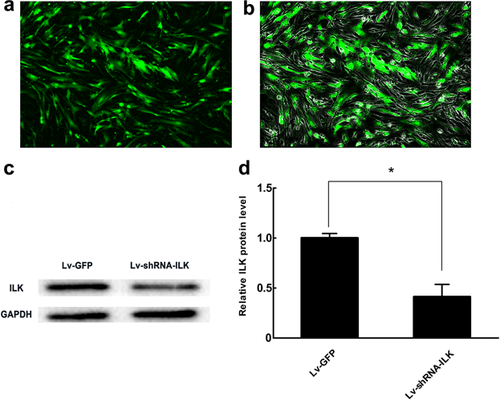
The shRNA targeting ILK and negative control sequences were ligated into the GV118 vector mU6-MCS-Ubi-EGFP. After the different fragments were combined with lentivirus vectors, they were transfected into 293T cells to package lentivirus particles.
Chondrocytes were divided into two treatment groups. Each group of cells was plated at a concentration of 105 cells/well into six-well plates and allowed to adhere for 1 day before transfection. Next day, the cells were transfected with Lv-shRNA-ILK vector or control Lv-GFP vector in serum-free medium. Twelve hours after transfection, all cultures were changed to complete medium. Three days after transfection, GFP expression was detected by fluorescence microscopy, and the expression of ILK protein was assessed by Western blot analysis.
Proliferation studies
Proliferation was assessed by two different methods, specifically, direct cell counting and the CCK-8 assay.
Direct cell counting
Cells were trypsinized and counted according to published protocols (Chaturvedi et al., 2007). Second-generation chondrocytes were seeded onto glass slides (25 mm × 25 mm) coated with type II collagen at a density of 105 cells/slide and randomly divided into different groups, each comprising six glass slides. Experiments were performed when cells had reached approximately 70–80% confluence. Chondrocytes were cultured for 3 days with or without periodic mechanical stress for 8 h per day, prior to direct cell counting. Cells were trypsinized and counted, and the cell number was determined by counting cells from each glass slide independently. Experiments were repeated five times.
CCK-8 assay
Cell proliferation was determined using CCK-8 solution according to the manufacturer's instructions (Dojindo Lab., Kumamoto, Japan) (Bae et al., 2010). Cells were added to 10 µL of CCK-8 solution in each well of five 96-well plates (n = 5) and incubated for 4 h at 37°C. The absorbance of each well was determined at 450 nm using a microplate reader.
Quantitative real-time PCR (qPCR) analysis
Total RNA was extracted using RNAiso Plus and reverse-transcribed into cDNA using the PrimeScript RT Reagent Kit, according to the manufacturer's protocol (TaKaRa). qPCR analysis was performed with the LightCycler System (Roche Diagnostics, Basel, Switzerland) using SYBR Premix Ex Taq II, as described previously (Kloesch et al., 2012). The reaction was performed in a 20 µL mixture containing 2 µL cDNA. Each cDNA sample was amplified using specific primers (Table 1, TaKaRa) under the following cycling conditions: 30 s initial denaturation at 95°C, followed by 40 cycles at 95°C for 5 s and 60°C for 20 s. Gene expression of AGC and Col2 was normalized against that for GAPDH.
| Genes | Sequence of primers (5′–3′) | AS (bp) | AT (degrees) |
|---|---|---|---|
| Aggrecan | F GAAGTGATGCATGGCATTGAGG | 146 | 60 |
| R ATGATGGCGCTGTTCTGAAGC | |||
| Type II collagen | F GAGGGCAACAGCAGGTTCAC | 95 | 60 |
| R TGTGATCGGTACTCGATGATGG | |||
| GAPDH | F GGCACAGTCAAGGCTGAGAATG | 143 | 60 |
| R ATGGTGGTGAAGACGCCAGTA |
- F, forward; R, reverse; AS, amplicon size; bp, base pairs; AT, annealing temperature.
Western blot analysis
Total protein was prepared and Western blot analyses were performed as described previously (Wang et al., 2007). Total protein was prepared using RIPA buffer, and the Bradford assay was employed to determine protein concentrations. Protein samples were resolved using SDS–PAGE and transferred to nitrocellulose membranes. Following blocking for 1 h with 5% skimmed milk in TBST, membranes were incubated with antibodies (1:1,000 dilution for all antibodies) overnight at 4°C. Blots were incubated with horseradish peroxidase-conjugated secondary antibody at ambient temperature for 1 h, and color was subsequently developed with ECL. Results were scanned using a gel imaging system (UVP LLC, Upland, CA, USA) and measured using Gel-Pro Analyzer software (Media Cybernetics, Rockville, MD, USA).
Statistical analysis
Statistical analyses were performed using SPSS 14.0 software (SPSS, Inc., Chicago, IL, USA), and results are expressed as mean ± standard deviation. Student's unpaired t-test and one-way analysis of variance (ANOVA), followed by post hoc Fisher's least significance difference (LSD) test, were used to determine statistical significance. A P-value <0.05 was considered significant.
Results and discussion
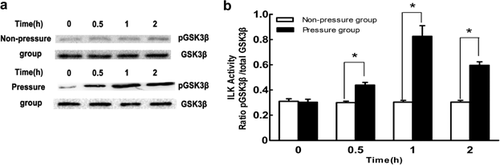
Periodic mechanical stress enhances ILK activity
ILK is widely considered to be an intracellular adaptor that couples integrins modulating multiple cellular responses (Bhattacharya et al., 2015; Moraes et al., 2015). Hsu et al. (2007) reported that ultrasound stimulation significantly enhanced the catalytic activity of ILK, leading to increased COX-2 expression in chondrocytes. However, the effects of mechanical stimulation on the catalytic activity of ILK in chondrocytes were unknown. We observed that exposure to periodic mechanical stress resulted in a rapid and sustained elevation of ILK activity (Figures 1a and 1b). The coincidence between ultrasound and periodic mechanical stress in the activation of ILK in chondrocytes raised the possibility that there may be some similar regulatory mechanisms between the two stimuli. Indeed, earlier studies by Schortinghuis et al. (2004) and Feril and Kondo (2004) elucidated that ultrasound stimulation could induce cavitation to bring out some biomechanical effects in cells.
ILK is required for periodic mechanical stress-initiated chondrocyte proliferation and matrix synthesis
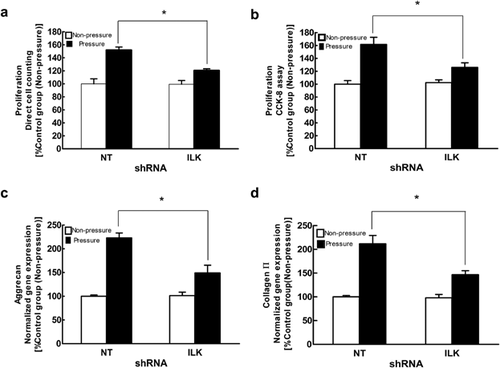
Next, we examined whether activation of ILK mediated periodic mechanical stress-induced chondrocyte proliferation and matrix synthesis. Several researchers have demonstrated that following various types of non-mechanical stimulation, ILK is essential for many cell behaviors and functions such as proliferation and development in chondrocytes (Grashoff et al., 2003; Terpstra et al., 2003). Our results revealed that 80% of chondrocytes were successfully transfected and that ILK protein exhibited a significant decrease in the Lv-shRNA-ILK group compared to the Lv-GFP group (Figures 2a and 2d). In addition, ILK prevention noticeably inhibited periodic mechanical stress-induced up-regulation of chondrocyte proliferation (Figures 3a and 3b) and matrix synthesis (Figures 3c and 3d). These results strongly suggest that ILK is a pivotal decision-making protein involved in chondrocyte functions in response to periodic mechanical stress. To the best of our knowledge, this is the first study to characterize the specific roles of ILK signaling in chondrocyte mitogenic effects following exposure to periodic mechanical stress.
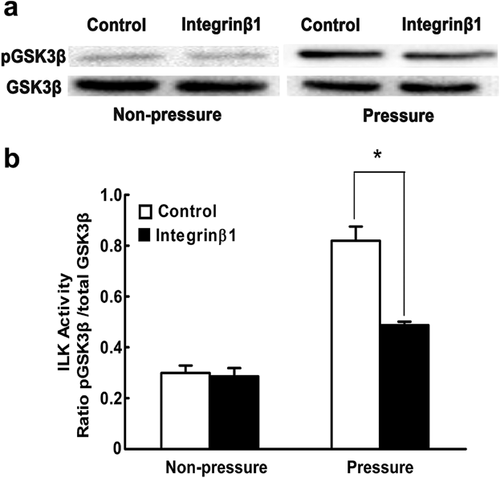
Integrin β1 is essential for periodic mechanical stress-induced ILK activity
We next aimed to examine the upstream and downstream relationships between ILK and integrin β1. ILK has generally been reported to be a pivotal integrin-associated signaling protein that transmits multiple extracellular inputs into intracellular biochemical signals (Pan et al., 2014; Yang et al., 2016). In our present investigation, we found that after pretreatment with a functional blocking antibody against integrin β1, periodic mechanical stress-induced ILK activity was markedly inhibited (Figures 4a and 4b). This result revealed that integrin β1 is located upstream of ILK in this context. Similarly, Nho et al. (2005) demonstrated that in a setting of matrix-derived mechanical forces, integrin β1 inhibition would result in significant depletion of the ILK activity responsible for fibroblast viability. However, some contrasting findings have also been reported. For instance, a study by Li et al. (2013) reported that in epidermal cells exposed to hepatocyte growth factor (HGF), silencing of ILK by RNA interference significantly blocked the expression levels of integrin β1 in the process of wound healing. Furthermore, in addition to integrins, some other cell surface receptors have been shown to regulate ILK in a variety of settings (Delcommenne et al., 1998). The differences may be due to the fact that signal transduction is a complex multi-component process and that variations in cell type, stimulus, and setting would contribute to different results. Based on our results, we concluded that the intrinsic ILK catalytic activity induced by periodic mechanical stress in chondrocytes is at least partly mediated by integrin β1, but that important roles for other receptors or signaling proteins upstream of ILK cannot be excluded.
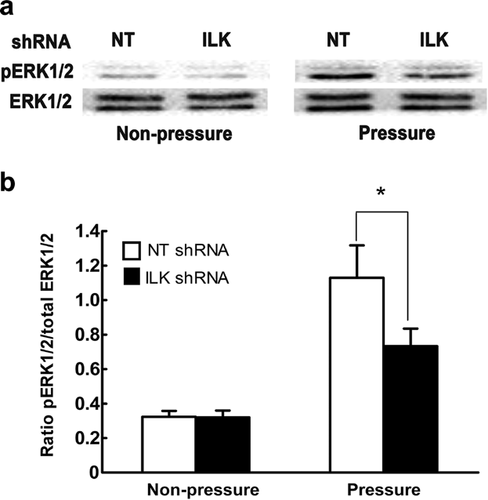
ILK modulates periodic mechanical stress-induced ERK1/2 phosphorylation
We then explored the regulatory roles of ILK in ERK1/2 phosphorylation under periodic mechanical stress. A search of the literature revealed an increasing number of publications indicating that activation of ERK1/2 is dependent on the activity of ILK when subjected to some non-mechanical stimuli in a variety of cell types (Naska et al., 2006; Tabe et al., 2007). However, the effect of ILK activation on ERK1/2 phosphorylation in mechanotransduction was poorly understood. We observed that the phosphorylation levels of ERK1/2 were obviously attenuated upon ILK inhibition by its targeted shRNA in response to periodic mechanical stress (Figures 5a and 5b). Our findings strongly imply a correlation between ILK and ERK1/2 activation in this context. In contrast, Maier et al. (2008) reported that although both ERK1/2 and ILK were activated by cyclic strain, ERK1/2 activation was not regulated by ILK in fibroblasts. One reasonable explanation for these differing results is that none of the complex signaling pathways act in isolation, and the same kinase could be regulated by various signal proteins while there is also crosstalk between pathways.
In conclusion, our study confirms that ILK is an indispensable regulator closely related to the integrin β1-ERK1/2 signal cascade responsible for chondrocyte proliferation and matrix synthesis under conditions of periodic mechanical stress.
Competing interests
The authors declare no competing interests.
Acknowledgements and funding
This work was supported by the National Natural Science Foundation (grant no.: 81271986).




