Novel role of zonula occludens-1: A tight junction protein closely associated with the odontoblast differentiation of human dental pulp cells
Abstract
Zonula occludens-1 (ZO-1), a tight junction protein, contributes to the maintenance of the polarity of odontoblasts and junctional complex formation in odontoblast layer during tooth development. However, expression and possible role of ZO-1 in human dental pulp cells (hDPCs) during repair process remains unknown. Here, we investigated the expression of ZO-1 in hDPCs and the relationship with odontoblast differentiation. We found the processes of two adjacent cells were fused and formed junction-like structure using scanning electron microscopy. Fluorescence immunoassay and Western blot confirmed ZO-1 expression in hDPCs. Especially, ZO-1 was high expressed at the cell–cell junction sites. More interestingly, ZO-1 accumulated at the leading edge of lamellipodia in migrating cells when a scratch assay was performed. Furthermore, ZO-1 gradual increased during odontoblast differentiation and ZO-1 silencing greatly inhibited the differentiation. ZO-1 binds directly to actin filaments and RhoA/ROCK signaling mainly regulates cell cytoskeleton, thus RhoA/ROCK might play a role in regulating ZO-1. Lysophosphatidic acid (LPA) and Y-27632 were used to activate and inhibit RhoA/ROCK signaling, respectively, with or without mineralizing medium. In normal cultured hDPCs, RhoA activation increased ZO-1 expression and especially in intercellular contacts, whereas ROCK inhibition attenuated the effects induced by LPA. However, expression of ZO-1 was upregulated by Y-27632 but not significantly affected by LPA after odontoblast differentiation. Hence, ZO-1 highly expresses in cell–cell junctions and is related to odontoblast differentiation, which may contribute to dental pulp repair or even the formation of an odontoblast layer. RhoA/ROCK signaling is involved in the regulation of ZO-1.
Abbreviations
-
- 4′DAPI
-
- 6-diamidino-2-phenylindole dihydrochloride
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- DMP-1
-
- dentin matrix protein 1
-
- DSPP
-
- dentin sialophosphoprotein
-
- GAPDH
-
- glyceraldehyde 3-phosphate dehydrogenase
-
- hDPCs
-
- human dental pulp cells
-
- LPA
-
- lysophosphatidic acid
-
- MLC
-
- myosin light chain
-
- qPCR
-
- real-time quantitative PCR
-
- ROCK
-
- rho-associated coiled-coil-forming kinase
-
- TJs
-
- tight junctions
-
- ZO-1
-
- zonula occludens-1.Introduction
Introduction
Columnar odontoblasts are connected by a junctional complex and consist of an ordered single layer located at the periphery of the dental pulp (Sasaki and Garant, 1996; Tjäderhane et al., 2013). The oriented arrangement of the odontoblast layer functions in dentinogenesis and the maintenance of tooth integrity (Arana-Chavez and Massa, 2004). The exposure of teeth to external stimuli, such as caries and attrition, may lead to injury of odontoblasts and to disruption of the junctional complex of adjacent odontoblasts (Arana-Chavez and Massa, 2004; Saito et al., 2013). Moreover, the remaining odontoblasts and regenerated odontoblast-like cells that differentiate from dental pulp cells secrete reparative dentin, to protect against external stimuli (Téclès et al., 2005). However, the structure of newly formed reparative dentin is quite different from that of primary dentin. Reparative dentin exhibits a random orientation, and the junctional complexes formed between odontoblast-like cells are also disordered (Arana-Chavez and Massa, 2004). It seems that the junctional complex is related to the oriented arrangement of the odontoblast layer and to dentin structure.
The junctional complex consists of tight junctions (TJs), adherence junctions, and gap junctions. It has been confirmed that adherence and gap junctions appear in early odontoblasts, whereas TJs are associated with mature odontoblasts. After the assembly of TJs, odontoblasts can undergo terminal differentiation, thus becoming highly polarized and secreting matrix constituents for mineralization (Arana-Chavez and Katchburian, 1997; João and Arana-Chavez, 2003, 2004). Zonula occludens-1 (ZO-1), which was the first TJ protein to be identified, participates in the assembly of TJs, direct or indirect binding to gap junctions or adherence junctions, early mammalian embryogenesis, tissue organization, and the wound-healing process (Taliana et al., 2005; Puller et al., 2009; Huo et al., 2011). As a major regulator of TJ assembly, ZO-1 is widely expressed in epithelial and endothelial cells in vertebrates (Guillemot et al., 2008). Moreover, ZO-1 may participate in the mediation of transcellular transport, the maintenance of the polarized distribution of epithelial cells (Fanning, 2006), and the regulation of the functions of TJs as barriers and fences (Hartsock and Nelson, 2008). ZO-1 was also detected in nonepithelial cells, such as cardiomyocytes and fibroblasts (Duffy et al., 2004; Hunter et al., 2005). During tooth development, differentiation of odontoblasts is associated with the formation of the junctional complex. Gap and adherence junctions are present in the early stage of differentiated odontoblasts, and tight junctions assemble during odontoblast maturation (Arana-Chavez and Katchburian, 1997). Therefore, gap juctions, adherence junctions, and tight junctions are all related to the differentiation of odontoblasts in tooth development. As an actin cytoskeleton-binding protein, ZO-1 is associated with tight junction, gap junction, and adherence junction. Between adjacent odontoblasts, ZO-1 is abundantly located (João and Arana-Chavez, 2003, 2004) and a decrease in ZO-1 expression results in the disruption of cell junctions and abnormal arrangement of odontoblasts in mice (Lee et al., 2009; Jiménez et al., 2011). It suggested that ZO-1 plays an essential role in the maintenance of odontoblast layer. In the reparative process of pulp–dentin complex, the communication and signaling transduction through cell–cell junctions between dental pulp cells might participate in regulating migration and odontoblast differentiation. And as an important junctional protein, ZO-1 might play a role in this process. However, there is lack of studies indicated the expression of ZO-1 in human dental pulp cells (hDPCs). In addition, it remains unclarified whether ZO-1 is related to the reparative process of pulp–dentin complex.
ZO-1, a cytoplasmic junctional protein that is closely related to actin filaments, can duty for scaffolds to assemble TJs’ integral membrane proteins, actin cytoskeletons, and cytosolic proteins (Hurd et al., 2003; Roh and Margolis, 2003). Meanwhile, RhoA and its major effector Rho-associated coiled-coil-forming kinase (ROCK) mainly regulate cytoskeletal remodeling by mediating the stress fibers to the extracellular matrix (Riento and Ridley, 2003). Hence, ZO-1 might be regulated by RhoA/ROCK pathway. Our previous study confirmed Lysophosphatidic acid (LPA) activated RhoA and Y-27632 specifically inhibited ROCK (Cheng et al., 2010), thus we used LPA and Y-27632 in the present study to activate or inhibit RhoA/ROCK signaling. Two odontoblast differentiation markers, dentin sialophosphoprotein (DSPP) dentin matrix protein 1 (DMP-1), were used to measure the differentiation of hDPCs in vitro.
In the present study, we confirmed the expression of ZO-1 in hDPCs explored the relationship between ZO-1 and odontoblast differentiation in vitro and investigated the potential role of RhoA/ROCK signaling in the regulation of ZO-1.
Materials and methods
Cell culture and treatment
Sample selection was approved by the ethics committee of the West China Hospital of Stomatology, Sichuan University. Human third molars were extracted from adults (aged between 18 and 25 years), and informed consent was obtained from the participants. Human dental pulp cells (hDPCs) were isolated and cultured according to a method described previously (Cheng et al., 2010). Cos-7 cells were cultured as the control according to reported method (Huo et al., 2011). Cells between passages 3 and 6 were used. hDPCs were treated with 50 μg · mL−1 ascorbic acid (Sigma–Aldrich, St. Louis, MO), 10 mM β-glycerophosphate (Sigma–Aldrich, St. Louis, MO), and 100 nM dexamethasone (Sigma–Aldrich, St. Louis, MO) for 7, 14, and 21 days, to induce odontoblast differentiation (Chen et al., 2013). hDPCs were treated with 0 or 5 μM lysophosphatidic acid and/or 0 or 10 μM Y-27632 (Cheng et al., 2010, 2011).
Transfection
Cells were seeded to 30–50% confluence in 6-well plate with Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY) containing 10% FBS without antibiotics. Twenty-four hours later, the ZO-1 shRNA were diluted in serum-free DMEM according to the manufacturer's instruction and the medium was used to perform the transfection. The medium was replaced with 10% FBS plus DMEM after eight hours. Cells were thus used in the following experiment after 24 h incubation. The target sequence of ZO-1 shRNA was as follows: GCGTCTCTCCACATACATTCT. The target sequence of the control was TTCTCCGAACGTGTCACGT.
Immunofluorescence
hDPCs were plated on a 24-well plate for 24 h and fixed with 4% paraformaldehyde for 20 min, followed by treatment with 0.25% Triton X-100 for 5 min at room temperature. After blocking with 10% goat serum for 1 h, cells were incubated with a mouse anti-ZO-1 antibody (Invitrogen Life Technologies, Carlsbad, CA) for 60 min at 37°C and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Molecular Probes, Inc., Eugene, OR) for 5 min at room temperature. Cells were viewed on a fluorescence microscope (Carl Zeiss, Göttingen, Germany).
Scanning electron microscopic analysis
hDPCs were seeded on glass cover slips for 24 h and fixed with 2.5% glutaraldehyde for 2 h at 4°C. Subsequently, the cells were dehydrated serially through ethanol rinses (30, 50, 70, 95, and 100%), followed by critical-point drying with liquid carbon dioxide and coating with gold (Varalakshmi et al., 2013). Samples were observed under a scanning electron microscope (Inspect F50, FEI).
Western blot analysis
To analyze cellular protein levels, cells were scraped and extracted for Western blot analysis according to a method described previously (Cheng et al., 2011). Ice-cold lysis buffer was used to lyse the cells and cell lysates were centrifuged at 12,000g for 10 min at 4°C. The supernatants were then subjected to SDS/PAGE (10% gel). Proteins were transferred to polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA). Membranes were then blocked with 5% bovine serum albumin (BSA) for 1 h. The primary antibodies including rabbit anti-ZO-1 (1:1,000; Cell Signaling Technology, Inc., Danvers, MA), DSPP (1:1,000; Santa Cruz, CA), DMP-1 (1:1,000; Abcam, Cambridge, MA), and rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:1,000; Epitomics, Burlingame, CA) and the membranes were incubated overnight at 4°C. The secondary antibody was horseradish peroxidase-conjugated anti-rabbit IgG (1:10,000; Santa Cruz, CA) and the membranes were incubated for 1 h at room temperature. An enhanced chemiluminescence kit (Millipore) was used to visualize the immunoreactive bands. And the bands were analyzed using Adobe Photoshop CS5 software (Adobe Systems, San Jose, CA).
Wound-healing assay
A wound-healing assay was performed to induce migration. hDPCs were seeded onto a 24-well plate for 24 h and cells were starved for 24 h. Subsequently, cells were scratched with a P200 pipette tip. After 18 h, cells were fixed with 4% paraformaldehyde for immunocytochemical detection of ZO-1 in migrating cells.
Alizarin red staining
Cells cultured for 0 or 21 days were fixed with 4% paraformaldehyde for 20 min at room temperature. Then, cells were stained with 40 mmol/L alizarin red S (PH 4.2, Sigma–Aldrich, St Louis, MO) for 10 min at room temperature (Varalakshmi et al., 2013). After washing, the mineralized nodules were observed using an optical microscope.
Real-time quantitative PCR (qPCR)
Total RNA was extracted from hDPCs using the TRIzol reagent according to the manufacturer's instructions (Invitrogen Life Technologies, Carlsbad, CA). cDNA was synthesized using iScript and was then used in quantitative PCR reactions with SYBR Green. The primer sequences used were as follows: 5′-GAGACTGGTTCCATGGCCTC-3′ and 5′-CCACCAAATGCACAACGTACT-3′ for ZO-1; 5′-TCACAAGGGAGAAGGGAATG-3′ and 5′-TGCCATTTGCTGTGATGTTT-3′ for DSPP; 5′-AGTGCCCAAGATACCACCAG-3′ and 5′-CATTCCCTCATCGTCCAACT-3′ for DMP-1; and 5′-CTTTGGTATCGTGGAAGGACTC-3′ and 5′-GTAGAGGCAGGGATGATGTTCT-3′ for GAPDH. GAPDH was used as a control. Reactions were performed on an ABI 7300HT apparatus.
Statistical analysis
Each experimental condition was repeated at least three times. Differences were analyzed using a one-way analysis of variance. The significance level was set at P < 0.05.
Results
ZO-1 expressed in hDPCs and especially high expressed in cell–cell junctions
ZO-1 expressed in the cytoplasm and cell membrane of hDPCs, as assessed using immunofluorescence staining (Figure 1A). Western blot analysis confirmed the expression of ZO-1 in hDPCs compared with the positive control (P < 0.05) (Figure 1B). The processes of adjacent hDPCs were fused and formed a cell–cell junction-like structure (Figure 1C). Moreover, ZO-1 was particularly located at cell–cell junctions between adjacent cells (Figure 1D).
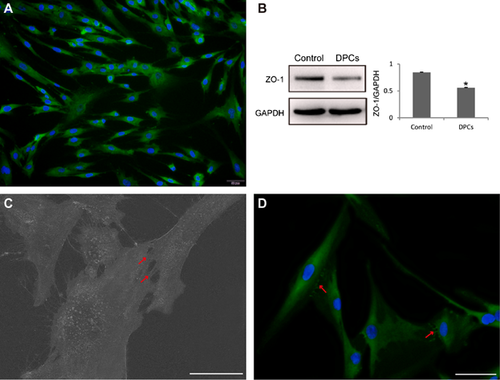
ZO-1 was related to the repair process of hDPCs in vitro
As cell migration is one of the initial steps in the repair process of the pulp–dentin complex, we tested the expression of ZO-1 in migrating hDPCs. Cells migrated for 18 h in an in vitro wound-healing assay compared with the control (Figures 2A1 and 2A2). Immunofluorescence detection of ZO-1 in migrating cells showed that ZO-1 was localized at the leading edge of cell lamellipodia (Figure 2A3). This indicated that the positional expression of ZO-1 might be related with the hDPC directed migration observed during the wound-healing process.
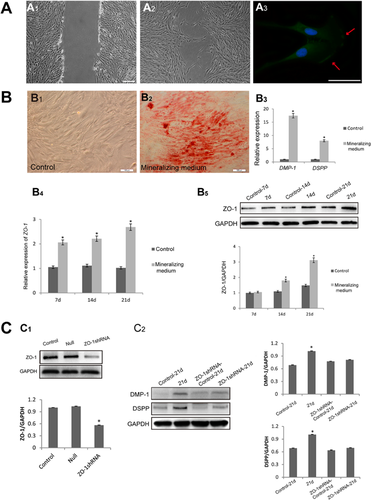
DSPP and DMP-1 are two significant markers of odontoblasts (Arana-Chavez and Massa, 2004). Therefore, we detected the expression of DSPP and DMP-1 to measure the odontoblast differentiation of hDPCs. After cells were cultured for 21 days, wide and large mineralized nodules were formed and the relative expression of DSPP and DMP-1 was obviously increased at the genetic level (Figures 2B2 and 2B3). Interestingly, ZO-1 expression increased gradually with the time of culture in mineralizing medium, both at the gene (Figure 2C1) and protein level (Figure 2C2). To confirm the relationship between ZO-1 and odontoblast differentiation, ZO-1-shRNA was used and the effectiveness was detected. Figure D1 showed ZO-1 was significantly inhibited (P < 0.05). The silence of ZO-1 greatly decreased the DMP-1 and DSPP protein expression when cells were cultured by using mineralizing medium for 21 days (Figure 2D2). This showed that ZO-1 is closely related to the odontoblast differentiation of hDPCs.
RhoA/ROCK pathway was involve in ZO-1 regulation
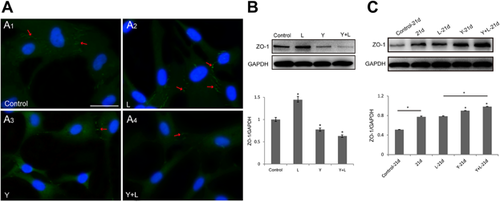
RhoA activation induced by LPA promoted ZO-1 expression, especially in cell–cell junctions (Figure 3A2, arrow) compared with the control (Figure 3A1); however, the inhibition of ROCK by Y-27632 led to a decrease in ZO-1 expression between cells (Figure 3A3, arrow). ROCK inhibition lowered the expression of ZO-1 (Figure 3A4, arrow) induced by RhoA activation (Figure 3A2). Besides, Western blot analysis showed that the total expression of ZO-1 can be regulated by RhoA/ROCK signaling (Figures 3B and 3C). In Figure 3B, LPA significantly increased ZO-1 while Y-27632 inhibited the expression (P < 0.05). In addition, the previous Y-27632 treatment inhibited the LPA inducement and greatly decreased ZO-1(P < 0.05). Interestingly, the modification pattern of RhoA/ROCK was different after 21 days’ mineralized culture of hDPCs. It revealed that Y-27632 increased the ZO-1 expression of significance (P < 0.05); however, there was no statistically significance between LPA group and the control (Figure 3C).
Discussion
In the present study, we described a novel phenomenon: ZO-1 expression was confirmed in hDPCs and especially highly expressed in cell–cell junctions and at the leading edge of lamellipodia in migrating cells. More importantly, the expression of ZO-1 increased gradually after the odontoblast differentiation and the differentiation was inhibited due to the ZO-1 silencing. This suggested that ZO-1 plays an essential role in odontoblast differentiation during the process of dental pulp repair in vitro.
By binding to F-actin and transmembrane proteins (e.g., catenin and connexin) directly or indirectly, ZO-1 may regulate the assembly, stability, and function of cell junctions in nonepithelial cells (Fanning and Anderson, 2009; Chen et al., 2015). It also reported that the localization of adherens and gap junction at intercalated disks could be determined by ZO-1 via associating with N-cadherin multiprotein complex (Palatinus et al., 2011). In the present study, we confirmed the expression of ZO-1 in another nonepithelial cell line, hDPCs. And high expression of ZO-1 was detected in cell–cell junctions, which suggested that it might play a role in the communication between neighboring cells. Numerous studies have shown that cell–cell junctions, especially gap junctions, have an essential role in the intercommunication of adjacent cells. ZO-1 can bind directly to gap-junction-related proteins (such as connexin family), thus affecting the assembly and function of gap junctions and acting as a regulator of cell–cell communication (Laird, 2010). Hence, we supposed that ZO-1 might participate in substrate transportation and signaling transduction between hDPCs thus plays a role in the cell biological behaviors such as migration and differentiation in repair process of hDPCs.
hDPCs migrate to the injury site and differentiate into odontoblasts or odontoblast-like cells, followed by the secretion of matrix and mineralization into reparative dentin during the reparative process (Téclès et al., 2005). Thus, cell migration and differentiation are significant processes in dental pulp repair. We found that ZO-1 was particularly expressed at the leading edge of migrating cells, which was similar to that described in corneal fibroblasts in a previous study (Taliana et al., 2005). Lamellipodia, which constitutes a polarized dendritic actin array, maintains the orientation of the leading edge during cell migration (Andrew and Insall, 2007). Thus, the specific high expression of ZO-1 might contribute to the regulation of the directional migration of hDPCs. According to previous studies, the changes of gap junctions between cells might contribute to the repair of scrape wound. Wright et al. (2012) found Gap27 (connexin mimetic peptide) could increase human dermal fibroblast migration both in hyperglycemic and hyperinsulinemic conditions. Chen et al. (2015) reported that cerebral endothelial F-actin architecture and migration regulated by connexin 43/ZO-1 complex. Hence, the localization of ZO-1 might be attributed to gap junctions. We also found that the level of ZO-1 increased gradually together with the differentiation of hDPCs in vitro and the silencing of ZO-1 greatly decreased the DMP-1 and DSPP expression. The observation of the gradually increasing with odontoblast differentiation might indicate that changes of cell junctions and the rearrangement occurred during odontoblast differentiation and maturation. On the other hand, decreasing level of ZO-1 may inhibit this process, thus restrain the differentiation of hDPCs. However, further studies are needed to explore these issues.
ZO-1 binds directly to actin filaments and transmembrane proteins via PDZ domains to regulate cytoskeleton dynamics (Schneeberger and Lynch, 2004). The interactions between ZO-1 and the actin cytoskeleton regulate TJ-mediated permeability and the reorganization of the junctional complex (Terry et al., 2010). RhoA regulates actin cytoskeleton organization by phosphorylating the myosin light chain (MLC) and LIM kinase through ROCK (Riento and Ridley, 2003). Some studies reported that RhoA signaling modulates TJ integrity and barrier properties in endothelial cells (Adamson et al., 2002; Baumer et al., 2008). The present study showed that Y-27632 decreased ZO-1 expression, especially at cell contacts. This result was similar to a previous study that reported that ROCK inhibition disorganized the apical ring of actin to decrease tight junction formation, thus resulting in the decrease of ZO-1 in epithelial cells (Pipparelli et al., 2013). RhoA phosphorylates LIM kinase via ROCK to maintain actin cytoskeleton stabilization (Riento and Ridley, 2003). Inhibition of ROCK may disrupt the stability and influence the binding between ZO-1 and F-actin in undifferentiated hDPCs. However, ROCK inhibition surprisingly increased the expression of ZO-1 after mineralized culture of hDPCs. This suggested that the inhibited cytoskeleton by Y-27632 might lead to disruption of junctional components initially and the new cell–cell junctions and/or even rearrangement of cells would be induced during the odontoblast differentiation thus ZO-1 expression was upregulated. RhoA activation can be induced by LPA via Gα12/13; moreover, our previous study showed that LPA can activate RhoA in hDPCs (Cheng et al., 2010; Xiang et al., 2013). Here, we found that the expression of ZO-1, which was localized at cell–cell boundaries, was increased after RhoA activation; however, ROCK inhibition prevented this effect in undifferentiated hDPCs. These results were consistent with those of a previous study that reported that RhoA activation could better maintain the junctional structures (Gopalakrishnan et al., 1998). It also revealed that pre-treatment of Y-27632 to inhibit ROCK would block the RhoA activation by LPA. In odontoblast differentiated hDPCs treated by LPA, the level of ZO-1 remained unchangeably. This may indicate that some other signaling pathways were involved in the differentiated process. But it still needs further studies to confirm this hypothesis.
Conclusions
In conclusion, ZO-1 expressed in hDPCs, especially high expressed at cell–cell junctions and the leading edge of migrating cells. The gradual increase of ZO-1 expression followed with odontoblast differentiation and silencing of ZO-1 greatly inhibited differentiation indicated that ZO-1 is related with hDPC odontoblast differentiation. RhoA/ROCK signaling pathway was involved in regulating of ZO-1; however, the mechanisms still needs further studies to clarify. Our study implies that ZO-1 may contribute to the repair process of the pulp–dentin complex, or even the formation and arrangement of the odontoblast layer.
Acknowledgments and funding
This work was supported by the National Natural Science Foundation of China (No. 81371134, 81400504, and 81500846).
Conflict of interest
The authors deny any conflicts of interest related to this study.




