Optimization of porcine urothelial cell cultures: Best practices, recommendations, and threats
Abstract
Many experimental approaches have been conducted in order to isolate urothelial cells from bladder tissue biopsies, but each method described has utilized different protocols and sources of bladder tissue. In this study, we compared the different methods of urothelial cell isolation available in literature together with standardized methods in order to obtain more unified results. Five methods for primary porcine urothelial culture establishment were compared: tissue explants and four enzymatic methods utilizing collagenase II, dispase II, combination of dispase II and trypsin, and trypsin alone. The average number of isolated cells, cell morphology, success of established culture, average number of cells from the first passage, expression of p63 and pancytokeratin and the characterization of urothelial cell growth, and aging were analyzed during the in vitro culture. The method utilizing dispase II was the most efficient and reproducible method for the isolation and culture of porcine urothelial cells when compared to the other tested methods. Urothelial cells obtained by this method grew considerably well and the cultures were established with high efficiency, which enabled us in obtaining a large quantity of cells with normal morphology. Contamination with fibroblasts in this method was the lowest. The utilization of a proper method for urothelial cell isolation is a critical step in the urinary tract regeneration when using tissue engineering techniques. In summary, this study demonstrated that by utilizing the described method with dispase II, a suitable number of cells was achieved, proving the method useful for tissue regeneration.
Abbreviations
-
- BPE
-
- bovine pituitary extract
-
- DMEM
-
- Dulbecco's modified Eagle medium
-
- EDTA
-
- ethylenediaminetetraacetic acid
-
- FBS
-
- fetal bovine serum
-
- HBSS
-
- Hank's balanced salt solution
-
- IRS
-
- immunoreactive score
-
- MTT
-
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
-
- PBS
-
- phosphate-buffered saline
Introduction
The creation of the urinary bladder wall substitute in vitro requires a large number of cells. Therefore, the elaboration of efficient methods for the isolation and culture of the urothelial and smooth muscle cells was an essential factor in determining the success of this method (Kloskowski et al., 2013). One of the most important features of the urothelium is its high proliferative potential and the ability to self-renew. Therefore, in many studies, urothelial regeneration in the tissue engineered urinary bladder was not analyzed. However, it should be noted that urothelial cell seeding on the inner surface of the scaffold could prevent a urine leakage to the deeper layers of the graft and avoid the damage to the smooth muscle or stem cells (Atala, 2001; Drewa et al., 2004; Nagele et al., 2008; Pokrywczynska et al., 2014a,2014b).
In an attempt to resolve for the lack of a standardized approach for primary urothelial cell culture establishment, we tested and compared five different techniques (Lorenz et al., 1996; Kreft et al., 2002; Southgate et al., 2002, Fu et al., 2011; http://cellntec.com/wp-content/uploads/pdf/Isolation_Bladder.pdf). We provided some modifications to each method in order to obtain more unified conditions, which helped with further comparison of obtained results. The aim of this study was to develop an efficient and reproducible method for the isolation and culture of porcine urothelial cells.
Materials and methods
For the isolation of urothelial cells, 10 male domestic pigs (∼129 kg) were used. All experiments were approved by the Polish Local Ethical Commission, permission no. 32/2013.
Immediately after the bladders were harvested, they were immersed in the DMEM/Ham's F12 medium (Dulbecco's modified Eagle medium, HyClone, USA) supplemented with antibiotics (penicillin 100 U/mL, streptomycin 100 μg/mL, amphotericin B 5 μg/mL, Sigma–Aldrich, Germany). Each bladder was transferred into the petri dish, exposed and opened longitudinally with a pair of scissors. Then, the region of the trigone was cut off and excluded from further procedures. After that, the bladder was transferred into distilled water for a minute and placed into a fresh medium supplemented with antibiotics. Under sterile conditions, the urothelium was separated from the underlying stroma and the smooth muscle layer. The isolated tissue was cut into pieces (1 cm2). Suture markers were used to mark the urothelial surfaces. The obtained tissue pieces were washed five times in phosphate-buffered saline (PBS, HyClone, USA). Then, they were placed in the DMEM/ Ham's F12 medium supplemented with 10% Fetal Bovine Serum (FBS, Sigma–Aldrich, Germany) and antibiotics (penicillin 100 U/mL, streptomycin 100 μg/mL, amphotericin B 5 μg/mL, metronidazole 100 μg/mL).
The urothelial cell isolation
Five methods described previously by other authors (tissue explants and four enzymatic methods), with a few modifications (Table S1), were compared in order to elaborate an efficient and reproducible method for the isolation and culture of porcine urothelial cells. A total of 175 isolations (35 isolations/method) were performed. To exclude an interindividual variability, we compared the yields of 25 isolations from each bladder (5 isolations/method/bladder).
Method 1
Each explant (2–3 mm2) was properly oriented on a petri dish, so that the urothelium was on the bottom side. Then, the explants were incubated without the culture medium for ∼20 min, until attachment. After that, 5 mL of CnT-58 medium (Cellntec, Switzerland) was added.
Method 2
Tissue pieces (1 × 1 cm) were incubated for ∼15 h at 4°C in a conical tube containing 5 mL of stripping solution (Hank's Balanced Salt Solution [HBSS, Sigma–Aldrich, Germany] enriched with HEPES [1%, PAA, Austria], aprotinin from bovine lung [1 KIU- 27.75 ml, Sigma–Aldrich, Germany] and EDTA [9%; Polish Chemical Reagents, Poland]). Then, using the blunt side of a scalpel, the urothelial surface was mechanically scraped from the tissue piece. The obtained cell suspension after centrifugation (350g, 5 min) was resuspended in 2 mL of collagenase II solution (100 U/mL, Gibco, USA). The scraped cells were digested with shaking for 20 min at 37°C. After the collagenase inactivation, the cell suspension was centrifuged at 350g for 5 min and the obtained cell pellet was resuspended in the culture medium.
Method 3
Tissue pieces (1 × 1 cm) were digested overnight (∼15 h) at 4°C in dispase II (Gibco, USA) and dissolved in HBSS (2.4 U/mL). After enzyme inactivation, the urothelium was carefully scraped with the blunt side of a scalpel and the remaining tissue was discarded. Then, separated from conglomerates, urothelial cells were centrifuged at 350g for 5 min. After supernatant discarding, the cell pellet was resuspended in culture medium.
Method 4
Tissue pieces (1 × 1 cm) were digested with 5 mL of dispase II (Gibco, USA) in HBSS (2.4 U/mL) for 30 min at 37°C with low-speed shaking. After inactivation, the urothelial cells were separated from the underlying tissues by gently scraping, using the blunt side of the scalpel. After the residues of the pieces were removed, the scraped cells together with the solution were centrifuged. The obtained pellet was digested with 2 mL of trypsin (0.25%, Biomed, Poland)/EDTA (0.02%, Polish Chemical Reagents, Poland) solution for 15–20 min at 37°C with shaking. After enzyme inactivation and centrifugation of the cell suspension (350g, 5 min), the cell pellet was resuspended in the culture medium.
Method 5
Tissue pieces (1 × 1 cm) were incubated overnight (∼15 h) at 4°C in 0.25% trypsin/0.02% EDTA. After enzyme inactivation, the urothelial cells were gently scraped off and the tissue residues were excluded from further sampling. The obtained cell suspension was centrifuged for 5 min at 350g and resuspended in the culture medium.
Cell culture
The isolated cells were seeded at a density of 4 × 104 cells/cm2 and cultured in the CnT-58 growth medium (CELLnTEC, Switzerland). The culture medium was changed every 2–3 days. The cells were cultured in standard conditions at 37°C with 5% CO2 and 98% humidity. Cultured cells were detached from the growth surface with the use of accutase solution (0.13 mL–1 cm2) (Sigma–Aldrich, Germany).
Success rate of primary culture
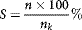
nt – total number of established cultures.
nk – number of cultures established successfully.
Histological/cytological and immunohistological/immunocytological analysis
The cell pellet obtained after the first passage was resuspended in 100 μL of alginic acid (1.5%, Sigma–Aldrich, Germany). Drops of suspension were carefully added into the petri dish with calcium chloride (0.1 M, Polish Chemical Reagents, Poland). “Alginate beads” were left in the calcium chloride until their gelation. Then, they were fixed with buffered formalin solution (10%). Urinary bladder wall fragments (1 × 1 cm) and urinary bladder tissue pieces after preparation were also analyzed. The fragments were fixed with buffered formalin solution (10%).
Hematoxylin–eosin staining
Staining with hematoxylin–eosin was automatically performed in the Shandon Varistain 24-4 apparatus (Thermo Scientific, USA).
Immunohistochemical/immunocytochemical staining
Antigenic determinants were exposed by heating in the Target Retrieval Solution buffer (pH = 9, Dako, Denmark) in a water bath for 40 min at 95–99°C. Endogenous peroxidase activity was inhibited by incubation in 3% H2O2 for 10 min. The addition of 5% BSA (Sigma–Aldrich, Germany) blocked the non-specific binding of antibodies. Then, the tissue slides were incubated with primary monoclonal antibodies against the p63 protein (Roche, Switzerland), against pancytokeratin and against the smooth muscle actin (SMA, Abcam, USA). After that, incubation with secondary antibodies was performed (EinVisionTM+ FLEX anti Mouse/Rabbit HRP, Dako, Denmark). Antigen–antibody complexes were visualized using 3.3′-diaminobenzidine (DAB(+) Chromogen, DAB(+) Substrate Buffer; Dako, Denmark).
Levels of the analyzed marker expression were established on the basis of the 5-point IRS scale (immunoreactive score) by Remmele (Remmele and Stegner 1987): (1) <10%; (2) 10–50%; (3) 51–80%; (4) >80% positive cells. We also took into account the 3-point representativeness of the specimens as follows: (1) low-cell concentration; (2) middle-cell concentration; (3) rich-cell concentration.
Characterization of urothelial cell growth in vitro
Urothelial cells from the first passage were seeded in 24-well culture plates at the density of 4 × 104 cells/cm2 per well. Cell viability was measured using the MTT assay and the trypan blue exclusion test.
Urothelial cells aging
Cellular aging was analyzed using the β-Galactosidase Staining Kit according to the manufacturer's instructions (Clontech, USA). As a negative control, the established 3T3 mouse fibroblast cell line was used. The percentage of aging cells was calculated by the use of Photoshop software (Photoshop CC, USA).
Statistical analysis
Comparisons of the obtained results were made between the groups using ANOVA with the NIR post hoc test. Data were analyzed by the SPSS Statistica (SPSS, USA). The significance level P < 0.05 was used as a reliable measure.
Results
Histological and immunohistochemical analysis of the urinary bladder wall
Hematoxylin and eosin staining of the urinary bladder sections confirmed the presence of urothelial, smooth muscle, submucosal and mucosal layers (Figures S1a and S1e). The positive expression of the p63 (Figures S1b and S1f) and pancytokeratin (Figures S1c and S1g) was observed in the bladder urothelium, while expression of SMA was visible in the smooth muscle layer (Figures S1d and S1h). In the urinary bladder wall fragments, after the mechanical separation, the presence of well preserved urothelium and residues of the bladder smooth muscle were observed (Figures S1e–h).
Isolation efficiency
The average number of cells isolated using different methods was presented in Figure 1a. The results showed that the highest number of cells was provided by Method 3 (22.9 ± 5.8 × 105 cells, P < 0.001), whereas Method 2 provided the lowest number of isolated cells (6.1 ± 1.7 × 105 cells). Cell numbers provided by Method 4 was comparable to Method 5 (17.5 ± 4.0 × 105 and 1.8 ± 4.4 × 105 cells, respectively; P > 0.1).
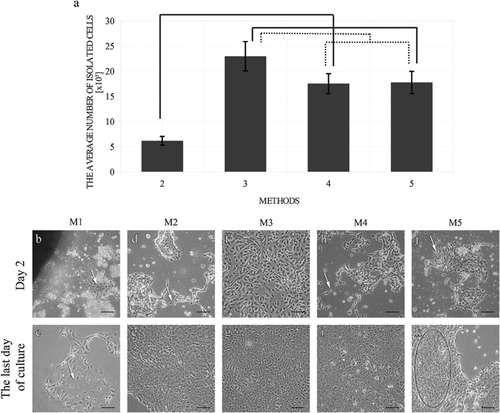
Cell morphology
The morphological analysis of primary urothelial cell cultures is presented in Figures 1b–k) and Table S2.
The average number of urothelial cells from the first passage
The tissue explant method (Method 1) resulted in low numbers of urothelial cell colonies. Primary culture establishment utilizing this method was not efficient. Cultures did not reach confluence and were not passaged. The average cell numbers from the first passage were comparable between Methods 3, 4, and 5 (P > 0.1). The Method 2 resulted in a five- to sixfold decrease in cell number from the first passage (1.4 ± 0.4 × 106 cells) in comparison to the other enzymatic methods (Figure S2a). The cells from Method 3 were passaged the earliest occurring on the 5th day following the cell seeding (Figure S2b).
Success rate of established primary cultures
The most effective methods for the establishment of a primary urothelial cell culture were those utilizing the dispase II (Method 3) and the combination of dispase II and trypsin/EDTA (Method 4) (Figure S2c). More than 80% (Method 4) or even 90% (Method 3) of the primary cultures established using these methods were successful. The Method 5 (tryspin/EDTA) had a success rate of about only 50%. All cultures established from the tissue explants (Method 1) were unsuccessful, similarly, like the majority of the primary cultures established using the collagenase II (Method 2). In consequence, techniques utilizing the tissue explants and the digestion with collagenase II were the least effective methods in this study.
Cell phenotype
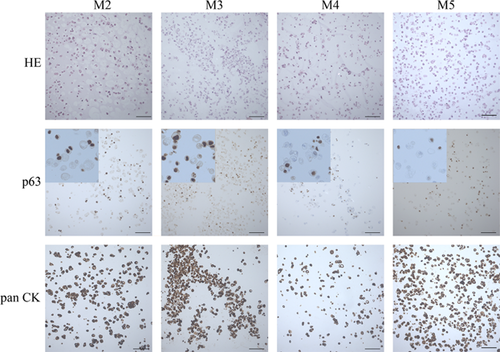
The expression of the p63 protein and pancytokeratin was well visible in all established cell cultures (Table S3, Figure 2). Pancytokeratin expression confirmed the epithelial origin of isolated cells, while the p63 protein expression revealed the presence of potential urothelial stem cells (basal cells). The level of both p63 and pancytokeratin expression in urothelial cells was comparable between all methods (IRS = 6 for p63 and IRS = 12 for pancytokeratin) with the exception of Method 4 in which lower expression of pancytokeratin was observed (IRS = 6 for both p63 and pancytokeratin).
Cell growth and viability
The growth curve of urothelial cells was determined with the MTT assay and the trypan exclusion test (Figures S3a and S3b). After 2 days of culture, the urothelial cells displayed an adaptation phase (lag) and entered a logarithmic growth phase (log) which finished after 5–6 days of culture. Afterwards, the cells entered the stationary growth phase (plateau).
Cell aging
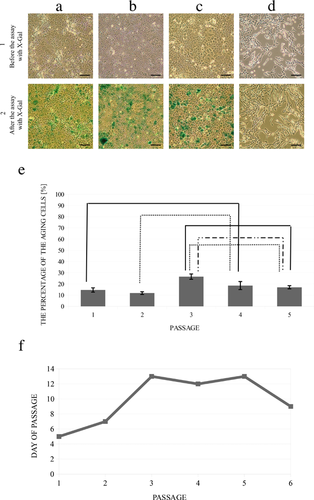
Analysis of ß-galactosidase expression confirmed the presence of cell aging in the in vitro culture (Figure 3). The percentages of aging cells from the first and the second passages were comparable (P > 0.1). The number of aging cells increased significantly after the third passage and was maintained at a comparable level up to the fifth passage (Figure 3e). The 3T3 mouse fibroblasts were negative for the expression of β-galactosidase, which confirmed the accuracy of the performed test (Figure 3d). Urothelial cells started to change their morphology at later passages. Cells from the fifth passage were slightly larger than cells from the earlier passages (Figures 3a–c). The growth rate also changed. Before the first and the second passage, the cells reached confluence on the 5th–7th day. In the following passages, confluence was not made until the 9th day of culture (Figure 3f).
Discussion
Several studies focusing on the establishment of primary urothelial cell cultures have been performed so far. Typically, only one enzymatic or direct explant technique has been evaluated in a single study without an answer about the effectiveness of the used methods when compared to other techniques proposed by different authors. In our study, the five different protocols used for the establishment of the primary cultures of urothelial cells were compared in order to develop an optimal, efficient, and repeatable method.
The commercially available Cnt-58 medium was selected and applied in all the experiments performed in this study. The selective criterion was a homogenous growth of urothelial cells. CnT-58 has been designed for urothelial cells and its use should limit the risk of contamination with fibroblasts. The cell culture in this medium did not require a feeder layer or a conditioned medium (CnT, Switzerland). However, the use of a selective medium significantly increases the costs of the experiment.
Compared to the other protocols, we did not obtain a large number of cells, having had a long culture period from tissue explants (Kreft et al., 2002). However, in the protocol described by Kreft et al., tissue explants of the mouse bladder were placed in culture inserts on 6-well plates. In our study, inserts were not used at all. Well orientating and spreading out of each piece is difficult, which could be the reason for the low number of urothelial colonies. The use of explants for urothelial cell establishment was not efficient in all attempts.
Culture establishment using Method 2 was unsuccessful, in most of the cases, despite the similar research model and methodology which was comparable to the other studies. (Southgate et al., 2002; Larsson et al., 2014).
The presented study showed that dispase II is the most efficient enzyme for porcine urothelium isolation. The transferring of urothelial conglomerates, recommended in the same protocol, turned out to be the best way to resolve the problem of contamination. In this method, the cell adhesion to the bottom of the culture vessel was improved in contrast to the other techniques where the whole suspension was centrifuged. Presumably the large number of unattached aggregates led to an increasingly slower attachment and proliferation of urothelial cells.
In this study, we obtained 1.8 × 106 cells from 1 cm2 bladder tissue using the trypsin/EDTA method. This result did not correspond with the number of cells isolated by Lorenz et al. (3.5 × 105). Other research models (porcine versus the sheep bladder) and the use of trypsin/EDTA, (0.25%/0.02%) in contrast to the 0.3% trypsin could be the reason of the better results.
Several authors showed the advantage of trypsin/EDTA over collagenase (Kurzrock et al., 2005). These results are consistent with our outcomes. However, based on the success rate of this method, it was still in comparison worse than those rates with the techniques using dispase II (Method 3) or the combination of dispase II and trypsin (Method 4). Lower success rates for Method 5 could be caused by the trypsin action. Trypsin over a shorter time period was also used in Method 4 (Method 4: 15–20 min, Method 5: the whole night). Several authors suggest that the overnight enzymatic digestion causes cell death (Ludwikowski et al., 1999). Our results did not confirm these findings. In our study, the results after an overnight digestion (Methods 3 and 5) were similar to those results after a short digestion period (Method 4).
The examined cells expressed p63 protein and pancytokeratin, which are characteristic urothelium markers (Ludwikowski et al., 1999). In the present study, the obtained results were comparable in most of the cases, except for Method 4, where the results were worse (Table S3, Figure 2).
Method 3 gave the best isolation efficiency and growth of urothelial cells in vitro (Table S3). Additionally, it was a repeatable method in contrast to Method 2 (collagenase II) and 5 (trypsin/EDTA). Method 4 (dispase II and trypsin/EDTA) also gave a high success rate, but cultures obtained from this protocol were characterized by a weak expression of cytokeratins (Figure 2). Dispase II alone or in combination with trypsin allowed for the ability to obtain comparable numbers of cells during the first passage (Figure S2a). However, cultures from Method 3 were passaged sooner when compared to the other methods (Figure S2b).
A limiting factor for the long-time culture is the senescence of cells. The presence of aging cells was examined in the confluent stage before every passage. Over 10% of the aging cells were observed in all tested cultures to the fifth passage (Figure 3). Ludwikowski et al. showed that pig and human urothelial cells can be cultured over a long time with no signs of senescence. However, in their study, they only analyzed the cell morphology after each passage without measuring the β-galactosidase activity (Ludwikowski et al., 1999). This was unlike our study, where the activity was measured.
Conclusions
The proper method of urothelial cell isolation is essential in obtaining the appropriate cell number from the smallest possible tissue fragment. The present study demonstrated that the enzymatic method using dispase II was the method of choice for urothelial cell isolation, which facilitated the ability to achieve better results when compared to the other methods tested in this study. Urothelial cells obtained in this way could be used for the regeneration of tissue-engineered urinary tract tissues and organs.
Acknowledgments and funding
The present work was supported by the National Center for Research and Development (NCBR) in Poland under Agreement no. STRATEGMED1/235368/8/NCBR/2014 (Smart AUCI Project) within the Strategic Programme STRATEGMED “Prevention practices and treatment of civilization diseases.”
Conflicts of interest
The authors declare that there are no conflicts of interest.




