β-Actin protein expression differs in the submandibular glands of male and female mice
Abstract
β-actin, a cytoskeletal protein, is the most widely used housekeeping gene. Although housekeeping genes are expressed in all tissues, the β-actin gene is expressed in certain cell types because of differential binding of transcriptional factors to the regulatory elements of the gene. The expression and localization of β-actin protein in the submandibular glands (SMG) of mice were investigated in this study, using Western blot analysis and immunohistochemistry. In ICR and C57BL/6J mice, the levels of β-actin protein in the SMG of females are significantly higher than those in the SMG of males. β-actin protein is majorly distributed in acinar cells of SMG. There is no significant difference in the expression level of β-actin protein between females and castrated males. After castrated male ICR mice are treated with 10 mg/kg/day testosterone propionate (TP) for 3 weeks, the levels of β-actin protein in SMG decrease. The numbers of duct per unit area increase, whereas the numbers of acinus per unit area decrease after TP administration. These data suggest that β-actin protein is mainly distributed in acinar cells of SMG and results in a marked sexual dimorphism in mice.
Introduction
Six actin isoforms have been identified in mammals: four muscle types (α-skeletal, α-cardiac, γ-enteric, and α-vascular) and two nonmuscle types (β-actin and γ-actin) (Mounier and Sparrow, 1997). The β-actin is one of two nonmuscle cytoskeletal proteins which are widely expressed in various tissues and participate in a variety of cell functions, such as maintenance of cell shape, cell migration, cell division, growth, and signaling (Heng and Koh, 2010; Bunnell et al., 2011). β-actin also plays a critical role in transcriptional regulation, mRNA transport, mRNA processing, and chromatin remodeling (Hofmann and de Lanerolle, 2006; Hofmann, 2009).
Because β-actin is ubiquitously expressed in nonmuscle cells, it is one of the most commonly used endogenous reference loading controls in laboratory techniques to normalize gene and protein expressions as it is believed to have constant expression levels in different cellular, experimental, and physiological conditions (Khan et al., 2014). However, recent studies have shown that the expression of β-actin can change in response to biochemical stimuli, during growth and differentiation, and in disease states (Ruan and Lai, 2007). Furthermore, differential expression level of β-actin among homologous tissues is detected. Yu et al. (2015) reported that the expression level of β-actin was lower in the proximal duodenum of mice, relative to the rest of the small intestines. β-actin expression was also shown to differ considerably with respect to brain area and gender in rats (Derks et al., 2008).
The basic structure of rodent SMG is constructed by embryonic organogenesis (Denny et al., 1997). The postnatal development of SMG is controlled under the neuronal and hormonal factors (Jacoby and Leeson, 1959; Gresik, 1980). The duct system of rodents is composed of intercalated, striated, excretory, and main excretory ducts. Saliva produced by acinar secretory cells in the glandular body flows sequentially through the intercalated, striated, and excretory duct (Amano et al., 2012). The differentiation of granular convoluted tubule (GCT) cells from striated ductal cells occurs during puberty under the control of hormonal factors, such as androgens and thyroid hormones (Chretien, 1977; Gresik, 1994; Adthapanyawanich et al., 2015). Androgens are considered as the primary factors in the differentiation of GCT cells because there is a marked sexual dimorphism in the development of GCT that proceeds around 3–5 weeks postpartum in the rodents (Chretien, 1977). Castration of adult male mice causes marked regression of GCT owing to conversion of GCT cells to striated ductal cells, whereas administration of androgens to castrated male or normal female mice readily causes the opposite phenomenon (Caramia, 1966; Chretien, 1977; Adthapanyawanich et al., 2015). Sexual dimorphism in the structure and function of SMG results in significant sex-related differences exist in the expression of many genes in mice (Treister et al., 2005).
β-actin was used as an endogenous reference loading control in our other studies; however, the levels of β-actin protein in SMG were different between male and female mice when loading same amounts of protein samples. In the present study, we investigate the expression and localization of β-actin protein in SMG and suggest that β-actin protein is majorly distributed in acinar cells of SMG and results in a marked sexual dimorphism in mice.
Materials and methods
Animals and treatments
ICR healthy mice at age of 7–8 weeks were obtained from Experimental Animal Center of Nantong University, China. C57BL/6J mice were purchased from Model Animal Research Center of Nanjing University, China. The mice were housed under standard conditions in our animal facility. The institutional animal ethics committee of Nantong University approved the use of animals for this study. All procedures were in accordance with the national guidelines.
In order to compare the level of β-actin expression in the SMG between male and female mice, four ICR or C57BL/6J males and four ICR or C57BL/6J females were used. For acute testosterone propionate (TP) exposure experiment, nine female ICR mice were randomly trisected with the name of control group, 12 h exposure group, and 24 h exposure group. The vehicle of corn oil and 10 mg/kg TP (0.1 mL/10g, dissolved in corn oil) were administrated by subcutaneous injections to mice in the control and two exposure groups, respectively. For chronic TP exposure experiment, (1) eight female ICR mice were equally and randomly divided into two groups according to their status of TP exposure. Each group of mice was reared for 3 weeks using subcutaneous injections of 10 mg/kg/day TP (exposure group) or corn oil (control group). (2) Eight male ICR mice were also equally and randomly divided into two groups after removing their testes. The mice in one group were exposed to TP, while those in another group were not. All of the mice were fed for 2 weeks after castration (in order to deplete endogenous androgen), followed by 3-week subcutaneous injections of 10 mg/kg TP (exposure group) or corn oil (control group).
Materials
TP, phenylmethanesulfonyl fluoride (PMSF), mouse anti-β-actin monoclonal antibody (A5316), and rabbit anti-GAPDH antibody (G9545) were purchased from Sigma–Aldrich (St. Louis, MO). Goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP) was purchased from Bioworld Technology Co. Ltd. (Minneapolis, MN). Novex ECL HRP chemiluminescent substrate Reagent Kit was purchased from Invitrogen (Carlsbad, CA). Peroxidase/DAB kit (K5007) was purchased from DAKO (Denmark). The Bio-Rad protein assay kit was purchased from Bio-Rad Laboratories (Hercules, CA). Complete EDTA-free protease inhibitor cocktail was purchased from Roche Diagnostics (Indianapolis, IN). All other reagents were purchased from a commercial supplier in their highest purity.
Western blotting
The SMGs of mice were isolated and homogenated in cold RIPA buffer (150 mM NaCl, 1.0% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris, pH 8.0, and EDTA-free protease inhibitor cocktail) by a glass homogenizer. After removing the tissue debris and nucleus by centrifugation at 10,000 rpm for 15 min at 4°C, the expression levels of β-actin and GAPDH in the supernatant were determined by Western blotting as described previously (Chen et al., 2014). Briefly, proteins were extracted from the SMG, and protein concentrations were determined by a Bio-Rad protein assay, using bovine serum albumin as a standard. The proteins were mixed with 2 × SDS sample buffer and denatured at 95°C for 10 min. Equal amounts of protein samples were separated by 10% SDS–PAGE after electrophoresis. The separated proteins were electrophoretically transferred onto polyvinylidene fluoride membranes in a mini-protein II Electrophoresis Apparatus (Bio-Rad). The blotted membrane was blocked with PBS containing 3% nonfat dry milk in 0.1% Tween 20 (0.1% T-PBS) at room temperature for 2 h and then incubated with each primary antibody at 4°C overnight. The dilution of primary antibodies, mouse anti-β-actin and anti-GAPDH, were both 1:50,000. The membranes were washed with 0.1% T-PBS and incubated with goat anti-rabbit or mouse IgG-HRP diluted 20,000 times at room temperature for 1 h and subsequently washed with 0.1% T-PBS. The membranes were then reacted with the ECL reagent, and exposed to an X-ray film during appropriate time. The bands on X-ray film were scanned and saved the file as TIFF format. Band intensity was quantified by the National Institutes of Health (NIH) ImageJ software and depicted in a graphical form.
Immunohistochemistry (IHC)
The SMG tissue was cut at the size of 5 mm3. The specimens were fixed at 4°C for 3 h, followed by a wash with PBS containing 20% sucrose at 4°C overnight. The specimens were then embedded in Tissue-Tec optimal cutting temperature compound and rapidly frozen in liquid nitrogen. The frozen sections of 5 µm thickness were cut with a Leica CM1950 cryostat. The slides were immersed in pre-cooled acetone for 10 min. After rinsed in PBS, the slides were incubated in 0.3% H2O2 solution in PBS at room temperature to block endogenous peroxidase activity. The slides were rinsed in PBS including 3% fetal bovine serum. After 1:5,000 diluted anti-β-actin monoclonal antibody was applied to the sections, the slides were incubated in a humidified chamber at 4°C overnight. Immunoreactive proteins were chromogenically detected with diaminobenzidine. The sections were counterstained with Harris’ hematoxylin and then dehydrated and mounted. To test the specificity of the primary antibody, control sections were stained simultaneously according to the same procedure, with the exception that the primary antibody was replaced with mouse serum (Burry, 2000). The images of stained sections were taken at 200× or 400× magnification under a light microscope (CX31, OLYMPUS, Japan) by a pathologist blinded to the study groups.
Hematoxylin–eosin (H&E) staining
The SMG tissues were fixed in 10% buffered formalin solution (pH 7.2) for 48 h. After fixation, the material was subjected to the process of dehydration, diafanization, and inclusion in paraffin. For each animal, five nonconsecutive histological sections and 6 μm thick cross sections of the fragments were used. After hematoxylin–eosin staining, the histopathological change was observed under a microscope (CX31, OLYMPUS, Japan). The images of stained sections were taken at 200× magnification.
Statistical analyses
All data were presented as mean ± standard deviation (SD). Statistical significance was assessed by the independent sample t-test using SPSS software packages (SPSS version 17.0 for Windows; SPSS, Inc., Chicago, IL). Differences were considered significant at a P < 0.05.
Results
Sexual dimorphic expression of β-actin protein in mice SMG
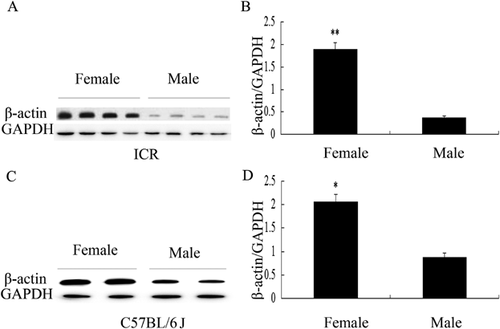
First, we determined the levels of β-actin expression in SMG between male and female ICR mice. In this study, GAPDH was used as an endogenous control of β-actin in the quantitative analysis of Western blot. We conducted repeated experiments to demonstrate that GAPDH was uniformly expressed in SMG of male and female mice. Similar result was reported in the study of Hipkaeo et al. (2008). Western blotting data revealed that the expression levels of β-actin protein in SMG of female ICR mice were significantly higher than that in male ICR mice (P < 0.01) (Figures 1A and 1B). To better understand the difference of the levels of β-actin protein in SMG between male and female mice, another mice strain C57 BL/6J was checked. Similarly, higher levels of β-actin protein were detected in SMG from female C57 BL/6J mice (P < 0.05) (Figures 1C and 1D).
Localization of β-actin protein in SMG of mice
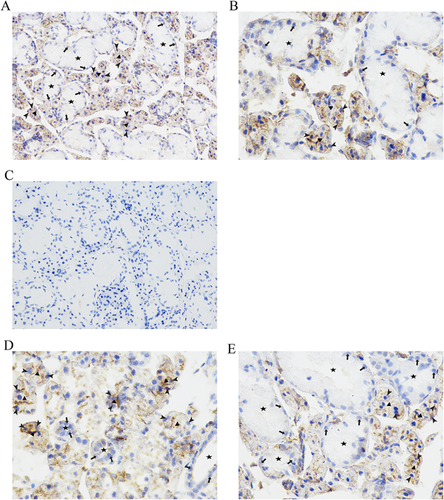
After detecting a higher level of β-actin protein in SMG of female mice than that of male mice, we studied the distribution of β-actin expression in different cell types of SMG tissues using IHC. In SMG tissues of mice, β-actin proteins were mainly localized in acinar cells, whereas ductal cells were weakly immunostained (Figures 2A and 2B). The specificity of the β-actin staining was confirmed when mouse serum was used to take place of anti-β-actin antibody (Figure 2C). The pattern of β-actin staining in the SMG of male mice was the same as that in female mice. The duct segments in the SMG of female mice (Figure 2D) were smaller and less numerous than those of male mice (Figure 2E).
The levels of β-actin protein in SMG of female ICR mice after chronic TP treatment
We further explored the underlying reasons for differential expression of β-actin in SMG between male and female ICR mice. Female mice were treated with 10 mg/kg TP. The levels of β-actin protein expression after 12 or 24 h exposure were detected by Western blotting. Our results showed that the level of β-actin protein expression was not changed after TP administration (Figure 3). These data indicated that androgen did not directly inhibit the expression of β-actin protein in SMG of ICR mice.

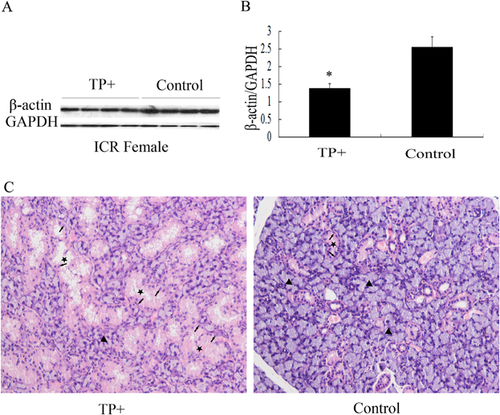
Then, we considered if TP could indirectly change the expression of β-actin in SMG of female mice. Female ICR mice were injected with 10 mg/kg/day TP for 3 weeks. The result of Western blotting revealed that the levels of β-actin protein expression in SMG of TP-treated mice were significantly lower than that in the control group (P < 0.05) (Figures 4A and 4B). The structure of SMG was altered in female ICR mice after exposure to TP. The results of H&E staining showed that GCT cells in the SMG of TP-treated female mice were larger with prominent pink granular cytoplasm than the cells in the SMG of normal female mice. The nuclei of the columnar GCT cells were located at the base of the cell. Pink secretary granules filled the cytoplasmic area between the nucleus and lumen of the duct (Figure 4C). The above results indicated that TP indirectly changed the levels of β-actin protein in SMG of female mice via altering the structure of SMG.
The levels of β-actin protein in SMG of male ICR mice after castration
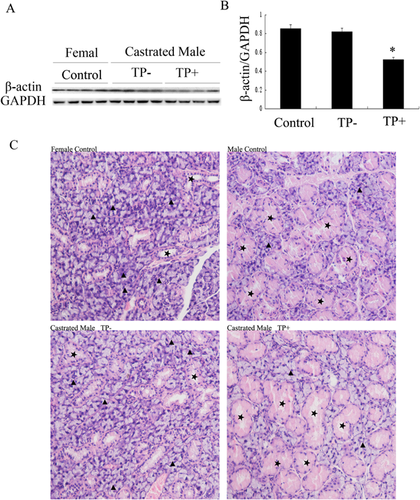
Next, we checked the levels of β-actin protein in SMG of castrated male mice. The difference in the levels of β-actin protein between castrated male mice and female mice was not statistically significant. However, the levels of β-actin protein in the SMG of castrated male mice treated with TP were much lower than that in the SMG of castrated males who were not treated with TP (P < 0.05) (Figures 5A and 5B). The results of H&E staining showed that castration of adult male mice caused marked regression of GCT, which was similar to normal female mice; whereas TP treatment caused hypertrophy of GCT in castrated males, which was similar to normal male mice (Figure 5C).
Discussion
In the present study, our data showed differential expression of β-actin in acinar and ductal cells of mice SMG. Owing to sex-related differences in the structure of mice SMG, the level of β-actin protein in SMG of mice is constitutively sexually dimorphic. Although the GCT cells in the ICR strain are larger than those in the C57BL/6J, BALB/c, and C3H/HeN strains, sex-related differences in GCTs are same in all mice strains (Miyaji et al., 2008). Our results indicate sexually dimorphic expression of β-actin in SMG of both ICR and C57BL/6J strains. β-actin is generally thought to be expressed in all cells of the organisms at similar levels because it is assumed that these genes are required for the maintenance of basic cellular function as constitutive gene. However, several data have shown that the level of β-actin expression varies depending on the developmental stage, type of tissue, and type of cell (Tokunaga et al., 1988; Lee et al., 2002; Lupberger et al., 2002; Kouadjo et al., 2007; Lin and Redies, 2012). Sex-related differences in the level of β-actin gene expression are observed in rat liver (Verma and Shapiro, 2006) and brain (Derks et al., 2008). The molecular mechanism of the difference in β-actin expression between ductal and acinar cells is unclear. CpG island hyper methylation of β-actin promoter has been found to be a negative regulator of expression (Quitschke et al., 1989). MiR-466a, miR-145, and miR-206 are known to target and degrade β-actin mRNA (Adams et al., 2007; Szczyrba et al., 2010; Sikand et al., 2012). Some upregulators of β-actin expression include matrigel, hormones, serum, hyperglycemia, hypoxia, and tumor necrosis factor-α (Ruan and Lai, 2007). The level of β-actin protein expression is different in acinar and ductal cells of mice SMG, presumably because of differential binding of transcriptional factors to regulatory elements of the gene.
The acinar cells of SMG secrete fluid and protein, such as mucins and amylase (Vreugdenhil et al., 1982). The final stage in exocrine secretion to translocate vesicles from their storage areas to the apical membrane is still unclear. Some studies show that actin-coated secretory vesicles of the pancreas and submandibular gland travel this distance over bundles of specialized actin cables emanating from the apical plasma membrane (Nemoto et al., 2004; Geron et al., 2013). The polarized distribution of β-actin to the apical surface and γ-actin to the basolateral plasma membrane is observed in gastric parietal cells (Yao et al., 1995). In the mouse pancreatic acinar cell, β-actin mRNA is expressed more than twofold greater than γ-actin (De Lisle and Robert, 2015). Therefore, β-actin is more likely to be important in acinar cells secretion process.
In conclusion, present study provides histological evidence of cell type-specific distribution of β-actin in ductal and acinar cells of mice SMG. The high level of β-actin protein in the SMG of female mice is due to more acinar cells in SMG per unit weight in females than in males. Therefore, β-actin is not suitable to be used as an internal standard when comparing male and female SMG gene expression. Further studies are needed to explore the relationship of cell type-specific differential expression of β-actin with its function.
Acknowledgments and funding
This work was supported by grants from the National Natural Science Foundation of China (21277078, 81302453, and 21347005).




