Umbelliferone and esculetin protect against N-nitrosodiethylamine-induced hepatotoxicity in rats
Abstract
N-nitrosodiethylamine (NDEA), a nitrosamine compound, is known to cause liver damage through the generation of reactive oxygen species (ROS), resulting in oxidative damage to macromolecules such as DNA, and the consequent development of cancer. The present study examines the protective effects of two antioxidant coumarin compounds umbelliferone (Umb) and esculetin (Esc) against NDEA-induced hepatotoxicity when administered in the diet to male Wistar rats. The results show that treatment with Umb (0.5% w/w) and Esc (0.5% w/w) in the diet for 7 days significantly attenuates NDEA-induced liver damage, lowering serum alanine transaminase (ALT) levels, decreasing hepatic lipid peroxidation, and restoring total glutathione levels. To investigate the mechanism for the observed protective effect, the levels of the key protective enzymes NAD(P)H: quinone oxidoreductase 1 (NQO1), heme oxygenase (HO1), and glutathione S-transferase Pi (GSTP1) were measured by Western blotting following Umb and Esc administration. The results showed that Umb and Esc administration significantly increased the expression of NQO1 by 3.6- and 2.7-fold, HO1 by 2.7- and 3.2-fold, and GSTP1 by 2.8- and 3.2-fold, respectively. In conclusion, Umb and Esc are capable of protecting liver from NDEA-induced hepatotoxicity, and this is associated with the induction of protective enzymes.
Abbreviations
-
- ALT
-
- alanine transaminase
-
- Esc
-
- esculetin
-
- NDEA
-
- N-Nitrosodiethylamine
-
- ROS
-
- reactive oxygen species
-
- Umb
-
- umbelliferone
Introduction
Reactive oxygen species (ROS) have been implicated in mediating the toxicity many compounds and other environmental insults such as ultraviolet light. In addition, ROS are known to contribute to various pathological conditions including cancer, neurodegenerative diseases, cardiovascular diseases, and aging. ROS, including superoxide anion, hydroxyl anion, and hydrogen peroxide, are produced in cells as by-products of normal metabolism, but can also be produced at higher levels by inflammatory processes.
ROS can attack lipids to give rise to reactive intermediates such as 4-hydroxynonenal, malondialdehyde, and acrolein (Ellis, 2007), and ROS and reactive lipid peroxidation products can cause significant damage to DNA, leading to mutation, and to the initiation of cancer (Marnett, 2000; Valko et al., 2006). In liver, ROS have been implicated in the initiation of hepatocellular carcinoma (HCC) through a range of mechanisms including inflammation, viral infection, and exposure to toxins, carcinogens, and alcohol (Schutte et al., 2009).
Models of oxidative damage in animals have been established using chemicals such as N-nitrosodiethylamine (NDEA; diethylnitrosamine; DEN). NDEA has been well characterized and shown to generate ROS through its metabolism, particularly in the liver (Bartsch et al., 1989; Bansal et al., 2005). This metabolism is carried out by members of the cytochrome P450 family and leads to the production of hydrogen peroxide, superoxide anion, and lipid-derived radicals (Farber and Gerson, 1984; Bartsch et al., 1989; Yamada et al., 2006). Treatment of animals with NDEA leads to increased levels of 8-hydroxyguanine within 72 h (Nakae et al., 1997), signifying oxidative damage to DNA, and this has been shown to be associated with the subsequent development of preneoplastic lesions within several weeks. NDEA is, therefore, commonly used as an initiating agent in models of carcinogenesis (Newell et al., 2008).
The involvement of ROS in cancer initiation has led to investigations into the potential for using antioxidant molecules as chemopreventive agents. Many antioxidant compounds have been reported to be beneficial against cancer initiation in both in vitro and in vivo models (Asamoto et al., 1990). These include a range of natural compounds including flavanoids, green tea polyphenols, and resveratrol (Khan et al., 2008; Stoner et al., 2008; Bishayee et al., 2010; Kim et al., 2010). Many of these compounds are thought to act through their ability to scavenge radicals, and therefore require very high doses for maximal beneficial effect. This precludes the use of most of these scavenger-based antioxidants in a therapeutic setting.
Coumarins are plant phenolic compounds that are found in dietary sources such as beans, legumes, essentials oils, and fruits from the Umbelliferae and Rutaceae families (Wu et al., 2009; Riveiro et al., 2010). More than 1,300 coumarins have been identified and are reported to exhibit a wide range of pharmacological activities, including antioxidant, anti-inflammatory, antitumor promoting, and antiproliferative properties (Kelly et al., 2000; Wu et al., 2009; Riveiro et al., 2010). Umbelliferone (7-hydroxycoumarin; Umb) and esculetin (6,7-dihydroxycoumarin; Esc) are coumarin derivatives with established antioxidant and free radical scavenging properties (Paya et al., 1992). Umb has been investigated as a treatment for diseases that display redox disturbances, including diabetes, inflammation and Parkinson's Disease, and at appropriate doses, shows significant therapeutic potential (Ramesh and Pugalendi, 2005; Vasconcelos et al., 2009; Barros et al., 2010; Subramaniam and Ellis, 2013). Esc has also been shown to protect cells from ROS damage (Kaneko et al., 2007; Kim et al., 2008; Barber et al., 2009; Subramaniam and Ellis, 2011). Our recent work in hepatocarcinoma cell lines (HepG2) has shown that Esc, in particular, prevents GSH depletion and protect cells from hydrogen peroxide-induced toxicity, through a mechanism that appears to be independent of free radical-scavenging ability (Subramaniam and Ellis, 2011). This makes both compounds particularly appealing because they appear to confer positive effects at very low, non-toxic doses. However, their ability to protect against ROS in liver in an animal model has not been previously investigated.
The aim of the present work was to examine the protective effects of Umb and Esc against NDEA-induced oxidative damage to liver in rats and to investigate the mechanism by which these compounds work, with a view to their use as chemopreventive agents, against toxicity and also in ROS-dependent pathological conditions.
Materials and methods
Animals
Male Wistar 10-week-old rats (Charles River, UK) were housed in cages in groups of three under controlled environmental conditions (19–23°C; 40–60% humidity; 12 h light/dark cycle) and acclimatized for at least 2 weeks.
Reagents
NDEA, umbelliferone, esculetin, butylated hydroxyanisole (BHA), and arachis oil were purchased from Sigma, UK. The primary antibodies NAD(P)H: quinone oxidoreductase (NQO1) and glutathione S-transferase Pi (GSTP1) were a gift from Professor John Hayes, University of Dundee. Heme oxygenase 1 (HO1) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies were procured from Santa Cruz Biotechnology, Germany.
Animal treatments and tissue preparation
After 2 weeks acclimatization, 11–12 weeks old male Wistar rats were divided into eight groups of six rats each. Two groups each were fed with normal diet containing 2% arachis oil (vehicle) or diet supplemented with test compounds Esc or Umb or BHA (positive control) in vehicle. The test compounds were provided in the diet for 7 days in the following amounts: BHA, 0.75% (w/w); Umb, 0.5% (w/w); and Esc, 0.5% (w/w). Animals were provided free access to food and water. Each rat on average consumed ∼30 g of the diet per day (with or without test compounds). Following 5 days of treatment, rats were administered with a single dose of NDEA at 200 mg/kg. After a further 2 days, the animals were sacrificed by cervical dislocation and the liver was removed immediately, sliced into two portions. One portion was used for histological studies as described below and the other portion was snap-frozen in liquid nitrogen and stored at −80°C until analysis. The snap-frozen tissue was homogenized in 20 mM sodium phosphate buffer containing 1 mM DTT (dithiothreitol, Sigma, UK), 100 µM EDTA (Sigma, UK), and protease inhibitor cocktail (Roche, UK). The homogenate was centrifuged at 10,000g for 30 min at 4°C and the supernatant was used for glutathione assay, lipid hydroperoxide assay, and Western blotting. The protein concentrations of the samples were measured by Bradford assay (Bradford, 1976).
Histological studies
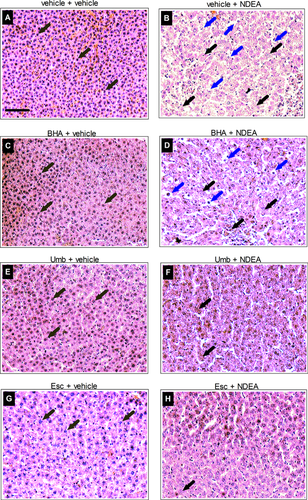
Liver tissue was collected as mentioned above, rinsed quickly in 0.1 M phosphate buffer saline and fixed in 4% paraformaldehyde (Sigma, UK) in 0.1 M phosphate buffer, pH 7.4, for 48 h. The fixed tissues were dehydrated with ethanol (70, 80, 90, and 100%, histoclear) and embedded in paraffin wax. Serial 10 µm-thick sections containing different lobes of the liver were cut into paraffin ribbons using a microtome (Leica, Inc.). The sections were then mounted on to the slides, air-dried for 30 min, and baked overnight in a oven at 45–50°C. The sections were deparaffinized, hydrated (100, 90, and 70% ethanol, distilled water), stained with hematoxylin solution for 3 min, and washed quickly in distilled water. Sections were dipped in 1% acid ethanol, washed with distilled water, and stained with 2% eosin for 5 min. Then, sections were rinsed with distilled water, dehydrated, and mounted using DPX. Hematoxylin stained liver images were examined under the microscope for morphological changes.
Lipid hydroperoxide assay
The assay for lipid hydroperoxide in liver extracts was performed using a kit according to the manufacturer's protocol (Lipid hydroperoxide assay kit, Cayman Chemical Company, USA, Cat. No.705002). Briefly, the kit measures the hydroperoxide levels directly utilizing the redox reactions with ferrous ions to produce ferric ions. The resulting ferric ions were measured using thiocyanate ion as the chromogen using spectrophotometer (Shimadzu, Inc.). A standard curve of OD500 versus concentration of lipid hydroperoxide standard was plotted and sample lipid hydroperoxide concentrations were calculated from the standard curve.
Measurement of total GSH levels
Total GSH in liver extracts was measured by an enzymatic recycling procedure which measures both oxidized GSSG and reduced GSH (Schafer and Buettner, 2001). Reduced GSH reacts with DTNB (5,5′-dithiobis-2-nitrobenzoic acid) to produce a yellow colored compound, 5-thio-2-nitrobenzoic acid (TNB). The rate of formation of TNB is measured at 412 nm, and GSH is quantified by reference to a standard curve. Any oxidized GSSG that is present is reduced to GSH by the presence of glutathione reductase, meaning that total GSH is measured.
A stock buffer of 100 mM sodium phosphate and 1 mM EDTA (pH 7.4) was used to prepare solutions of 0.4 mM NADPH, 4.5 mM DTNB, 1.9 U/mL GSH reductase (from Bakers Yeast, Sigma) and 5% sulfosalicylic acid (SSA). Standards of reduced GSH were prepared in 5% SSA. For each sample, three wells on the 96-well plate contained 170 μL reaction mixture (GSH reductase and NADPH), 20 μL liver homogenate or GSH standard, and 10 μL DTNB. The mixture was incubated for 10 min at 37°C and the absorbance was read immediately at 412 nm (Shimadzu, Inc.). The concentration of total glutathione in the samples was determined against the GSH standard calibration curve.
Determination of serum alanine transaminase activity
Rats were sacrificed at the end of the treatment and blood samples were collected. Serum was then separated by centrifugation at 3,000g for 5 min at 4°C and stored at −80°C for further analysis. The assay for alanine transaminase activity was performed according to manufacturer's protocol (Alanine transaminase activity assay kit, Cayman Chemical Company, USA, Cat. No.700260) using a spectrophotometer (Shimadzu, Inc.).
Western blotting
Tissue extract was prepared as mentioned in tissue preparation section above and diluted in two x Laemmli's sample buffer (LSB; Laemmli, 1970) and equal amounts of protein (10 μg) were loaded and separated on 10% polyacrylamide resolving gel (Laemmli, 1970). The resolved proteins were transferred to nitrocellulose membrane using a Bio-Rad Transblot apparatus for 1.5 h at 200 mA. Protein-binding sites were blocked with 5% BSA in TBST (20 mM Tris-HCl, 150 mM NaCl, 0.02% Tween-20) for 1 h. Nitrocellulose membranes were then incubated with antibodies against NQO1, GSTP1 (both antibodies were a gift from Professor John Hayes, University of Dundee), and HO1 (#SC7695, SCBT, Inc.) at 1:3,000 dilution overnight at 4°C. Membranes were washed thrice with TBST and incubated with secondary antibody (goat anti-rabbit horseradish peroxidase-conjugated, BioRad; 1:3,000) for 1 h and washed thrice with TBST. Antibodies were detected using enhanced chemiluminescence (ECL, Amersham). Quantification of band intensities was performed using NIH ImageJ analysis software.
Statistics
Statistical analysis was performed with SigmaPlot software (SigmaPlot 12.0, Systat Software, Inc., USA). Analysis of variance (ANOVA) was used to compare the drug treatment effects followed when appropriate by post hoc Bonferonni t tests versus “vehicle with NDEA treatment group” or “vehicle treatment group” as needed.
Results
Effect of dietary administration of Umb and Esc on NDEA-induced liver injury
Animals were treated with Umb, Esc, and BHA (a known protective compound) or vehicle control for 5 days before exposure to NDEA for 2 days. The effect of NDEA administration on liver cell architecture and integrity was examined by histological examination of liver sections. Liver tissue from NDEA-exposed animals (Figure 1B) showed significant loss of architecture and acute inflammatory cells with vacuolated cytoplasm indicating necrosis. However, in contrast, in the animals pretreated with Umb or Esc preceding NDEA administration, only mild necrosis and limited loss of cell architecture was observed (Figures 1F and 1H). BHA in the diet also reduced the damaging effects of NDEA but not to the same extent as Umb and Esc.
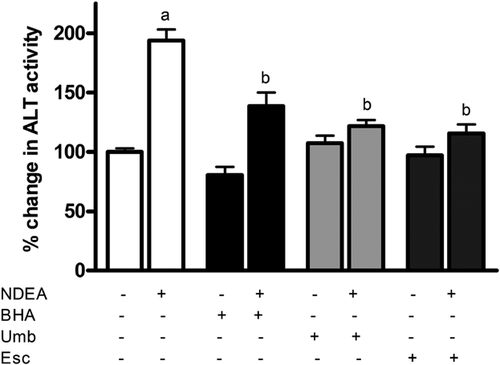
Levels of the liver enzyme alanine transaminase (ALT) were measured in the serum of NDEA-exposed rats as this enzyme is released following acute liver injury (Pratt and Kaplan, 2000). As expected, rats exposed to NDEA for 48 h showed a significant ∼2-fold increase in ALT levels compared to untreated control (Figure 2) indicating significant injury. However, dietary supplementation of Umb and Esc for 7 days preceding NDEA administration lowered the NDEA-dependent serum ALT levels by 80 and 87%, respectively, and were much closer to the levels seen in control animals. Dietary administration of BHA also lowered serum ALT levels but only by 57%. This indicates that Umb and Esc are more effective than BHA in protecting liver against NDEA damage at the doses used.
Effect of Umb and Esc on NDEA-induced lipid peroxidation
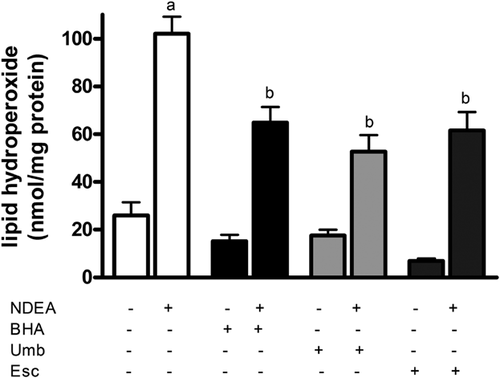
To understand how Umb and Esc are acting to protect liver cells against NDEA, levels of lipid hydroperoxide (LPO) in liver were measured as this represents the level of oxidative damage to cells. Administration of NDEA led to a marked fivefold increase in the levels of LPO in liver after 48 h compared to levels in livers from control animals (Figure 3). Rats fed with Umb and Esc supplemented diets significantly reduced NDEA-induced increase in LPO levels by 47% and 44%, respectively, compared to rats receiving vehicle alone. Treatment with the control compound BHA also lowered LPO levels but only by 37%. These results show that Umb and Esc are able to protect liver against lipid peroxidation induced by NDEA.
Effect of Umb and Esc on NDEA-induced GSH depletion in rat liver
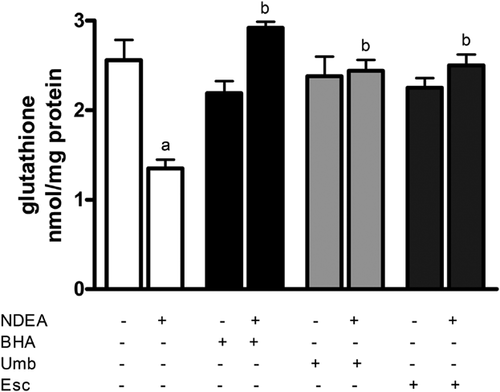
Total intracellular glutathione (GSH) was measured as an indicator of intracellular antioxidant depletion. As expected, administration of NDEA decreased GSH levels in rat liver extracts by 50% compared to liver from vehicle-treated control animals (Figure 4). However, Umb and Esc supplementation for 7 days prior to NDEA administration completely prevented NDEA-dependent GSH depletion in rat liver. A similar protective effect was observed in rats fed the BHA diet, indicating that all three tested compounds can protect rat liver against GSH depletion caused by NDEA administration.
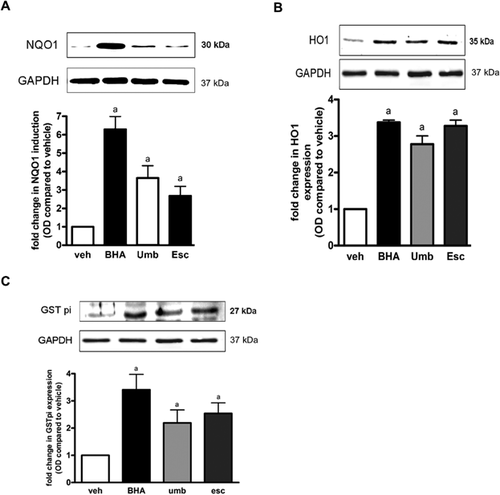
Effect of Umb and Esc on the expression of protective enzymes in rat liver
To further investigate the mechanism by which Umb and Esc protect liver from the toxicity of NDEA, the effect of the compounds on the induction of the protective enzymes NAD(P)H:quinoneoxidoreductase 1 (NQO1), heme oxygenase 1 (HO1), and certain glutathione S-transferases (GST) was investigated. These enzymes were chosen as previous studies have shown to be particularly sensitive to the redox state of the cell, and as such are considered biomarkers for sub-lethal oxidative stress within cells. The results in Figure 5 show that Umb and Esc administration significantly increased the expression of NQO1, HO1, and GSTP1 in rat liver. NQO1 expression was increased by 3.6- and 2.7-fold by Umb and Esc treatment, respectively. HO1 expression was increased by 2.7- and 3.2-fold by Umb and Esc, respectively, and GSTP1 levels were increased by 2.8- and 3.2-fold, respectively. The positive control compound BHA also increased the expression of NQO1 (6.2-fold), HO1 (3.3-fold), and GSTP1 (3.2-fold) significantly compared to vehicle-treated control. These results show that Umb and Esc are able to influence cellular antioxidant defenses in the liver and provide evidence for their mode of action.
Discussion
In this study, we have shown that dietary supplementation of two coumarins Umb and Esc to rats at low doses for 7 days significantly attenuates NDEA-induced liver damage, lipid peroxidation, and GSH depletion. It appears that Umb and Esc can augment the cell's antioxidant defense capability, and thereby help maintain GSH levels, and prevent the production of lipid hydroperoxides.
Esc has previously been reported to modulate the redox status of the cell and enhance GSH levels in mouse liver (Martin-Aragon et al., 1998) but the effect of low doses of Umb on GSH levels in rat liver has not been investigated previously. Umb and Esc are known to be potent-free radical scavengers, and some of their previously observed protective effects may be due in part to their ability to scavenge free radicals (Kanimozhi et al., 2011). However, our hypothesis was that Umb and Esc also lead to enhancement of the cell's enzymatic defense as indicated by our previous work showing that Esc can induce the expression of protective enzymes including NQO1 and HO1, in HepG2 cells (Subramaniam and Ellis, 2011), and can also convey neuroprotection to mice (Subramaniam and Ellis, 2013). In this study, we have significantly added to our earlier findings in showing that NQO1, HO1, and GSTP1 are inducible by Esc and Umb in the liver of rats. Taken together, these results indicate that induction of these enzymes may play a significant role in hepatoprotection againt NDEA and that low non-toxic levels of Esc and Umb are able to initiate this protection by induction of protective enzymes. These findings are significant, not only for protection against chemical toxicity, but also for ameliorating ROS-dependent pathologies that arise in liver. The dietary nature of these compounds is also appealing as food and drinks that are rich in Esc and Umb may also provide some protection against ROS from a range of sources, with wide-ranging health benefits.
It has to be noted that butylated hydroxyanisole (BHA), the compound we used as positive control in the current study, is reported to increase CYP2A6 levels in cultured monocytes (Jin et al., 2012) and this CYP2A6 induction by BHA might increase NDEA biotransformation in the liver, thus increasing its deleterious effects in this particular model. Perhaps that could be the reason for the lower protective effect of BHA compared to Umb and Esc, even though BHA showed the same inducing effect on HO1 and GSTP1, and even greater effect on NQO1 levels.
The induction of key antioxidant enzymes is considered to be part of an adaptive response to low sub-lethal levels of stress (Baird and Dinkova-Kostova, 2011). In this study, the low dose of Umb and Esc used is not sufficient to cause any observable toxicity to either animals or their liver cells, but is sufficient to induce a protective response. NQO1 encodes an NAD(P)H:quinone oxidoreductase, a two-electron reductase that prevents the one-electron reduction of quinones that releases ROS, and hence protects against oxidative stress (Jaiswal, 2000); HO1 encodes heme oxygenase 1, an enzyme involved in catabolizing-free heme and producing carbon monoxide, ferritin, biliverdin, and bilirubin, which mediate its antioxidant effects (Morse et al., 2009); and GSTP1 encodes a glutathione S-tranferase isoform, which is known to conjugate reactive aldehydes that are produced as a consequence of lipid peroxidation, and also activates the antioxidant peroxiredoxin VI (Ralat et al., 2006). Hence, all three of these enzymes have cytoprotective roles associated with elevated ROS, and their induction by low-level stress is concordant with this role. It is likely that other protective enzymes are also being induced in the liver as part of the stress response and that together these provide the observed level of protection conferred.
Previous studies have shown that induction of protective enzymes is mediated by the binding of the transcription factor Nrf2 to a specific DNA sequence present in the promoter of induced genes that is known as the antioxidant responsive element (ARE) (Rushmore and Pickett, 1990). It is known that other dietary compounds such as curcumin, anthocyanins, and melatonin also increase NQO1, HO1, and GST expression through an Nrf2-dependent mechanisms (Baird and Dinkova-Kostova, 2011) and that some are able to protect rats from chemical-induced liver injury (Farombi et al., 2008; Jung et al., 2009; Hwang et al., 2011). For example, an earlier study showed that the administration of coumarin to rats protected them from aflatoxin B1-induced hepatocarcinogenesis, and this was mediated through the induction of AKR7A1 (Kelly et al., 2000). However, our study is the first to demonstrate a clear association between Umb and Esc treatment, the induction of key protective enzymes, the maintenance of GSH levels, and significant protection against NDEA-induced liver damage.
Conclusions
In conclusion, the results presented show that Umb and Esc, natural compounds present in fruits and vegetables, protect against NDEA-induced hepatotoxicity in rats. The protective effect of these compounds is associated with the induction of Nrf2-dependent protective enzymes, and treatment with low-dose Umb and Esc leads to the maintenance of GSH levels and the prevention of lipid peroxidation. These dietary compounds, therefore, have the potential to protect against ROS-induced damage, and prevent the initiation of carcinogenesis.
Acknowledgments and funding
SRS was funded by an Overseas Research Scholarship and a University of Strathclyde Scholarship. We are grateful to Dennis McLoughlin for assistance with the histological analysis.
Conflict of interest
The authors declare that they have no conflict of interest.




