Injectable hydrogel delivery plus preconditioning of mesenchymal stem cells: exploitation of SDF-1/CXCR4 axis toward enhancing the efficacy of stem cells' homing
Abstract
Clinical applications of mesenchymal stem cells (MSCs) rely on their capacity to home and engraft in the appropriate target injury tissues for the long term. However, their homing efficiency has been observed to be very poor because of the lack or modifications of homing factors SDF-1α and CXCR4 receptors. Hence, this study was designed to investigate the homing and retention of pretreated human adipose tissue-derived MSCs (hASCs) from three different delivery routes in response to SDF-1α, released from chitosan-based injectable hydrogels. After stimulation of ASCs with a hypoxia mimicking agent, the expression level and functionality of CXCR4 were analyzed by flowcytometric analysis (FACS), transwell migration assay and qPCR. Then, the homing/retention of pretreated DiI-labeled hASCs were compared through three different in vivo delivery routes, 2 weeks after transplantation in Wistar rats. The cells were tracked histologically by fluorescent microscope and by PCR for human-specific CXCR4 gene. Results showed CXCR4 has dynamic expression pattern and pretreatment of hASCs significantly up-regulates CXCR4, leading to an increase in migration capacity toward 100 ng/mL SDF-1α in vitro and homing into the subcutaneously implanted hydrogel releasing SDF-1α in vivo. Furthermore, it seems that SDF-1α is particularly important in the retention of ASCs, in addition to its chemoattraction role. In summary, the delivery route in which the ASCs were mixed with the hydrogel rather than systemic delivery and local injection and preconditioning undertaken to increase CXCR4 expression concomitant with SDF-1α delivery by the injectable hydrogel, allowed for further homing/retention of ASCs. This might be a promising way to get better therapeutic outcomes in stem cell therapy.
Abbreviations
-
- AMD3100
-
- 1,1′-(1,4-Phenylenebis[methylene])bis-1,4,8,11-tetraazacyclotetradecane octahydrochloride
-
- BCIP/NBT
-
- 5-bromo-4-chloro-3-indolyl phosphate/ nitro blue tetrazolium chloride
-
- bFGF
-
- basic fibroblast growth factor
-
- BSA
-
- bovine serum albumin
-
- CH-GP-HEC
-
- chitosan-glycerophosphate-hydroxyethyl cellulose
-
- CoCl2
-
- cobalt chloride
-
- CXCR4
-
- chemokine (C-X-C motif) receptor 4
-
- DFX
-
- desferrioxamine mesilate
-
- DMEM
-
- Dulbecco's modified eagle (DME)-medium
-
- D-PBS
-
- Dulbecco's phosphate buffer saline
-
- FACS
-
- fluorescence activated cell sorting
-
- hASCs
-
- human adipose tissue-derived MSCs
-
- LiCl
-
- lithium chloride
-
- MMPs
-
- matrix metalloproteases
-
- MSCs
-
- mesenchymal stem cells
-
- SDF-1α
-
- stromal cell-derived factor-1 alpha
-
- SVF
-
- stromal vascular fraction cells
-
- VPA
-
- valproic acid
Introduction
Mesenchymal stem cells (MSCs) have been termed as promising stem cells for therapeutic applications and have been used in the clinic for the last decade (Wei et al., 2013), in part, because of their multiple beneficial paracrine effects (Chamberlain et al., 2007). Various sources of MSCs have been found (Wei et al., 2013) and among these human adipose tissue-derived MSCs (hASCs) are considered as a promising candidates for therapeutic purposes (Ahmadian Kia et al., 2011).
Regardless of beneficial properties and type of MSCs, routes of delivery, targeting and retaining of transplanted ASCs at the injured tissues are very crucial steps in describing the fate of tissue/organ regeneration (Chavakis et al., 2008; Law and Chaudhuri, 2013). There are several delivery routes for ex vivo-expanded MSCs including systemic infusion and local (direct) administration (Ankrum and Karp, 2010; Sarkar et al., 2011). It has been shown that ex vivo expanded MSCs, in both routes, are able to migrate/remain at sites of injury. Nonetheless, a significant barrier to the effective implementation of cell therapies is the inability to target a large quantity of viable cells with high efficiency in systemic delivery due to being trapped in the lung and spleen and cells escaping from the site of injection in local delivery (Chavakis et al., 2008; Ankrum and Karp, 2010).
Recently, chemokine receptors and their ligands, such as CXCR4, and stromal derived factor-1(SDF-1α), have been implicated in trafficking, homing, and engraftment of MSCs into and through tissue (Chavakis et al., 2008; Ahmadian Kia et al., 2011). It was shown that only a small fraction of MSCs express CXCR4, and response to SDF-1α-induced homing and expression of the CXCR4 decreases following sub culture (Rombouts and Ploemacher, 2003; Wynn et al., 2004; Sordi et al., 2005; Prockop, 2009). To address this challenge, some researchers have applied pretreatment to MSCs by using suitable pharmacological interventions, mimicking hypoxia, such as desferrioxamine mesilate (DFX), cobalt chloride (CoCl2), lithium chloride (LiCl), valproic acid (VPA), and physical hypoxia (Tang et al., 2009; Liu et al., 2010; Mirahmadi et al., 2013; Muscari et al., 2013). Several reports have suggested that some of beneficial effects of these pretreatments may be mediated by induction of chemokine receptors like CXCR4 on the cell surface (Tang et al., 2009; Liu et al., 2010; Tsai et al., 2010; Najafi and Sharifi, 2013). Thus, harnessing of this receptor would be a good strategy for increasing the homing of MSCs.
On the other hand, it seems that for directed homing, existence of SDF-1α (ligand of CXCR4) at an effective threshold and at appropriate time at the target tissue, which suffers from damage, is also important. SDF-1α level naturally increases after injury but falls back to baseline within a few days. More importantly, the exact duration of increase is not clear and there are inconsistencies among different tissues and organs (Zhang et al., 2008; Shimode et al., 2009). In our previous study, it was shown that chitosan-glycerophosphate-hydroxyethyl cellulose (CH-GP-HEC) is a good cytocompatible scaffold for drug delivery (Naderi-Meshkin et al., 2014a). Thus, local delivery of SDF-1α by this injectable hydrogel into an injured site may represent a promising therapeutic approach to enhance tissue regeneration via directing ASCs. In this way, SDF-1α, which has a very short half life (<15 min) and could be inactivated by matrix metalloproteases (MMPs; Segers et al., 2007), is delivered in a controlled and sustained manner by the scaffold. This would increase its effective half life, decrease its rapid diffusion into surrounding tissues and inactivation by MMPs (Naderi et al., 2011), and also produce a better gradient in an appropriate time for site-directed recruitment of stem cells to injured tissue (Bladergroen et al., 2009; Wang et al., 2010).
Therefore, in this study, a combined strategy, that is, (1) pretreatment of ex vivo expanded human ASCs, and (2) local delivery of SDF-1α, released from the CH-GP-HEC injectable hydrogels, was evaluated to investigate homing and retention of ASCs through three different cell-delivery routes (systemic administration, local injection, and encapsulated in scaffold). Based on our previous evidences that Xenotransplantation of human ASCs to rat is possible (Liechty et al., 2000; Sato et al., 2005; Haddad-Mashadrizeh et al., 2013b), we tested the hypothesis in rat animal model for simplicity.
Materials and methods
Cell isolation and culture
Discarded adipose tissues were obtained from healthy women, who underwent aesthetic liposuction. The washed lipoaspirates were treated with freshly prepared collagenase solution (1 mg collagenase type I [Invitrogen, Carlsbad, CA]), 10 mg of BSA (Biowest, Nuaillé, France) as carrier protein and 2 mM CaCl2 were dissolved in 1 mL PBS (for each 3 mL lipoaspirates) and incubated for 45 min at 37°C with gentle agitation in a shaker water bath. The mixture was centrifuged at 800 g for 10 min and the infranatant pellet (stromal vascular fraction cells; SVF) was separated and washed with PBS and re-centrifuged at 400 g for 6 min. The SVF pellet was resuspended and cultivated in low glucose Dulbecco's modified Eagle (DME)-medium (Biowest) containing 2 ng/mL basic fibroblast growth factor (bFGF, Royan Institute, Iran), 10% fetal bovine serum (FBS, Invitrogen), 100 µg/mL streptomycin and 100 U/mL penicillin (Invitrogen). Resuspended pellets from each of the 10 mL lipoaspirates were seeded in one 75 cm2 tissue culture treated flask. Medium was exchanged every 2–3 days and the cells became ∼90% confluent after 3–5 days. Then, the cells were trypsinized and replated into new flasks at a density of 5000–10000 cells/cm2. ASCs were characterized by morphological analysis, FACS pattern and standard differentiation assays. The cells at passage 3 were used for all experimental studies.
Study design and delivery routes of ex vivo expanded ASCs into animal model
After the general anesthetization of Wistar rats with ketamine and xylazine, contractually, in all experimental groups (five rats per each group) as shown in Figure 1 (groups A–G), 1 mL of CH-GP-HEC hydrogel containing SDF-1α (100 ng/mL) was injected subcutaneously on the right side of the rat's back and 1 mL of empty CH-GP-HEC hydrogel on the left side. In the meantime, DiI-labeled human ASCs (pretreated or untreated) were delivered from three different routes (Figure 1). The animals were treated and maintained according to the standard guidelines of Animal Care and Use Committee of Ferdowsi University.

Adipogenic and osteogenic differentiation
ASCs at passage 3 were induced to differentiate toward the adipogenic and osteogenic lineages according to the method of previous work (da Silva Meirelles et al., 2008). Briefly, the cells were seeded in six-well plates and grown to 80–90% confluency for adipogenic differentiation study. Adipogenesis was induced by culturing ASCs for 14 days in the adipogenic differentiation medium containing DMEM supplemented with 10% FBS, 200 µM indomethacin, 1 µM dexamethasone, and 10 mM β-glycerophosphate (all reagents were from Sigma–Aldrich, Munich, Germany). The differentiation medium was changed every third day and assessed using an Oil Red O stain (Sigma–Aldrich) as an indicator of intracellular lipid droplets and counterstained with Hematoxylin (Merck, White House Station, NJ). Osteogenesis was performed by inducing ASCs at 100% confluency for 21 days in an osteogenic medium containing DMEM supplemented with 10% FBS, 50 µM ascorbate-2-phosphate (Sigma–Aldrich), 0.1 µM dexamethasone, and 10 mM β-glycerophosphate. Osteogenesis was assessed by staining the calcium mineralization of extracellular matrix and alkaline phosphatase (AP) activity of osteoblast with Alizarin Red S (pH 4.1–4.3) stain (Sigma–Aldrich) and BCIP/NBT substrate (BD Bioscience, San Jose, CA), respectively.
Flowcytometric analysis
Flow cytometry was performed to characterize the ASCs and also to determine the expression of CXCR4 on the surface of both untreated and pretreated ASCs (passage 3). Cell cultures were prepared by trypsin/EDTA digest and washed twice with cold D-PBS, resuspended in D-PBS buffer containing 0.5% FBS at a concentration of 1 × 106 cells/mL. One hundred microliter (1 × 105 cells) of the suspension was transferred to a 1.5 mL microtube and was incubated with either respective antibody or isotype-matched control antibody. The cells were vortexed gently and incubated at RT for 45 min in the dark. Then, 400 μL of D-PBS buffer was added to each tube and the data was acquired by a flow cytometer (FACScalibur, BD Bioscience) and analyzed with Flowing Software 2.5 program.
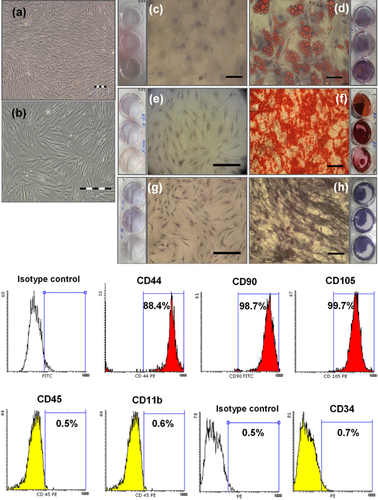
Antibodies used in flow cytometry analysis to characterize ASCs were rabbit anti-CD44 polyclonal antibody, rabbit anti-CD34 polyclonal antibody (all from antibodies-online, Aachen Germany), mouse anti-CD90 monoclonal antibody, rabbit anti-CD11b polyclonal antibody (all from Novus Biologicals, Littleton, CO), rabbit anti-CD105 polyclonal antibody, rabbit anti-CD45 polyclonal antibody (all from Bioss, Inc., Woburn, MA) and for analysis of CXCR4 expression (mouse anti-CXCR4 monoclonal antibody; from Neuromics, Edina, MN) on the surface of ASCs. For each antibody, respective isotype control was used.
Migration assay
ASCs chemotaxis assay was performed in 24-well plates based on the Boyden chamber principle using a polycarbonate membrane (8 µm pores size, Costar, corning, Tewksbury MA). ASCs were suspended in serum-free DMEM containing 0.5% BSA at a concentration of 25 × 104/mL. ASCs suspension at a concentration of 25 × 103 cells/mL in 100 μL was loaded onto the top wells, whereas 0.6 mL SDF-1α (100 ng/mL) was added to the bottom chamber. After 18-h of incubation at 37°C, 5% CO2, the non-migrating cells were completely wiped off from the top surface of the filters by cotton swab, and the migrating cells adhering to the undersurface of the filter membrane were stained with DAPI and counted. To demonstrate that ASCs migration toward SDF-1α is correlated with the upregulation of CXCR4, the cells were preincubated with AMD3100, a CXCR4-selective antagonist (De Clercq, 2003), at a concentration of 10 μg/mL for 30 min at RT. All experiments were performed three times in triplicate wells. At least five fields were counted from each well and the average number of migrating cells was determined.
Conventional and real-time PCR
Total RNA was extracted using Tripure reagent (Roche, Basel, Switzerland) according to the manufacturer's instructions. For all RNA samples, the quantity and purity were determined using the Nanodrop ND-1000 spectrophotometer (Nanodrop, BIO-TEK, Winooski, VT), integrity by gel eleterophoresis for 28S and 18S rRNA. Reverse transcriptase–polymerase chain reaction (RT-PCR) of CXCR4 and β-actin (housekeeping gene) was performed using 1 µg of total RNA for first strand cDNA synthesis. False positives and DNA contamination were controlled by omitting reverse transcriptase in control reactions. Conventional RT-PCR was performed to 35 cycles, 94°C for 30 seconds, 57°C for 40 s and 72°C for 30 s. Primers for RT-PCR were: β-actin (forward) GCTCAGGAGGAGCAAT (3′) and (reverse) GGCATCCACGAAACTAC (3′) with 187 bp product length; CXCR4 (forward) TCTGGAGAACCAGCGGTTAC (3′) and (reverse) GACGCCAACATAGACCACCT (3′) with 501 bp product length.
Quantitative real-time PCR analysis was performed using SYBR Green PCR Master Mix (Takara, Shiga, Japan) according to the manufacturer's instructions. Briefly, for relative quantification, we used a method that compares the amount of target normalized to a housekeeping reference gene (β-actin). Melting curve analysis was performed to check for a single amplicon. The formula used was 2−ΔΔCt, representing the n-fold differential expression of a specific gene in a treated sample compared with the control sample, where Ct is the mean of threshold cycle at which the amplification of the PCR product is first detected. Before using this method, we performed a validation experiment comparing the standard curve of the reference and the target to demonstrate that the efficiencies were approximately equal. The identity of the amplified products was checked by electrophoresis. The primer specific for real-time PCR was: CXCR4 (forward) ATCCCTGCCCTCCTGCTGACTATTC (3′) and (reverse) GAGGGCCTTGCGCTTCTGGTG (3′) with 231 bp product length. The primer sequence for β-actin was the same as for RT-PCR.
PCR was also performed, for tracking of hASCs from three different delivery routes, on DNA extracted from implanted hydrogels, lungs and spleen by human specific primer for CXCR4 gene. PCR of rRNA 18S was also used to prove infiltration of cells into the scaffold. Primers for PCR were: rRNA 18S (forward) ACTCAACACGGGAAACCTCA (3′) and (reverse) AACCAGACAAATCGCTCCAC (3′) with 123 bp product length; human specific CXCR4 (forward) GAGGAGTTAGCCAAGATGTGAC (3′) and (reverse) CCCATAGTGACTTCATTATATCCTTC (3′) with 116 bp product length.
Preparation of CH-GP-HEC thermosensitive injectable hydrogel
CH-GP-HEC injectable hydrogel was prepared according to our previous study (Naderi-Meshkin et al., 2014a). Briefly, the chitosan solution was obtained by dissolving 150 mg of chitosan (medium viscosity, 84% degree of deacetylated; Polysciences, Warrington, PA) in 9 mL of 0.1 M acetic acid solution, prepared in PBS and subjected to autoclave sterilization. To prepare the experimental mixtures, 1 mL of 1.3 M GP solution (filter-sterilized) was added dropwise to 9 mL of chitosan while stirred on ice for 15 min under aseptic conditions. Separately, HEC (Sigma–Aldrich) was dissolved at a concentration of 20 mg/mL in DMEM and sterilized by microfiltration. The SDF-1α (100 ng/mL) or cell pellet of ASCs (1 × 107 cells/mL) was homogeneously resuspended in 1 mL HEC, and then mixed with 4 mL chitosan-GP at 4°C. Immediately, the thermo-gelling cell suspension or/and SDF-1α-containing solution was injected into dorsum of rat (1 mL/injection site).
Histological assessment of transplanted animals
Fifteen days after transplantation, the rats were anesthetized (ketamine 50 mg/mL and xylazine 5 mg/mL, i.p.), perfused with 4% buffered paraformaldehyde (Haddad-Mashadrizeh et al., 2013) and the remaining implanted scaffolds were individually dissected and removed from the dorsum of rats. The tissues from lung and spleen were also removed. Immediately, half of all samples from each implanted scaffold, lung and spleen were processed for RNA analysis and the other half was fixed in 4% PFA, embedded in paraffin and used for histological analysis. Then, the embedded specimens were sectioned (4 μm) along the longitudinal axis of the implant, and the sections were sought for presence of DiI-labeled ASCs under an Olympus IX71 fluorescent microscope (Olympus, Tokyo, Japan) equipped with digital camera.
Statistical analysis
The SPSS v.21 statistical program was used to analyze the data. The values are reported as mean ± SD. Samples were obtained from at least three independent experiments and used to calculate the mean value. Significance of the data was examined in the level of P ≤ 0.05 using the one-way ANOVA and the Tukey test.
Results
ASCs characterization
Ex vivo expanded ASCs derived from lipoaspirates reached to passage 3 (P3) after 9–12 days of culture. They exhibited spindle-shaped morphology as shown in Figures 2A and 2B. Characterization of ASCs at P3 was performed by differentiating into mesenchymal tissues such as adipose (Figures 2C and 2D) and bone (Figures 2E–2H). MSC-derived adipocytes showed red lipid droplet by Oil Red O staining as compared with undifferentiated control. Undifferentiated MSCs (without extracellular calcium deposits) were slightly reddish by Alizarin Red S (Figure 2E) and showed weak alkaline phosphatase activity (Figure 2G), whereas MSC-derived osteoblasts (with extracellular calcium deposits) were bright orange-red (Figure 2F) and featured very high phosphatase activity (Figure 2H). In addition, ASCs were characterized by positive expression of CD44, CD105, CD90 and negative expression of CD34, CD45, and CD11b surface markers (Figure 2I).
CXCR4 has dynamic surface and intracellular expression
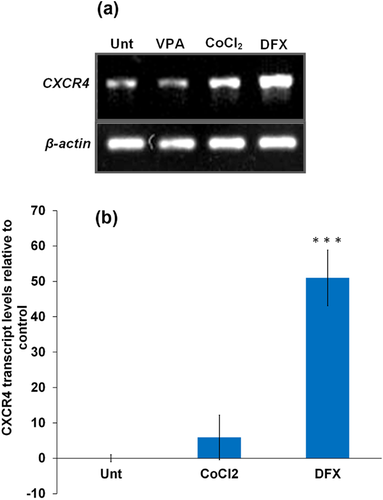
Flow cytometry was performed to detect the expression of SDF-1α receptor (CXCR4) on the surface of ASCs at passage 3 (Supplementary Figure S1). The results showed that CXCR4 could not be detected from extracellular staining of ASCs, even those pretreated with DFX, CoCl2, VPA, LiCl, and physical hypoxia; while 95.2% of a white blood cells population as positive control, expressed CXCR4 on their surface (Supplementary Figure S1). On the contrary, analysis of CXCR4 by in vitro migration assay showed that CXCR4 can be functionally represented on the cell membrane of DFX-pretreated ASCs to mediate SDF-1α-dependent homing within 24 h of reculturing after trypsinization (Supplementary Figures S2E and G). Furthermore, introduction of CXCR4 selective antagonist (AMD3100) has shown to significantly inhibit the chemotaxis of the ASCs toward SDF-1, confirming that SDF-1/CXCR4 interactions are responsible for the migratory response in DFX-pretreated ASCs (Supplementary Figures S2F and S2G). In addition, conventional and real time RT-PCR confirmed that ASCs express intracellular CXCR4 (Figures 3A and B).
Gelation time and degradation of CH-GP-HEC injectable hydrogel
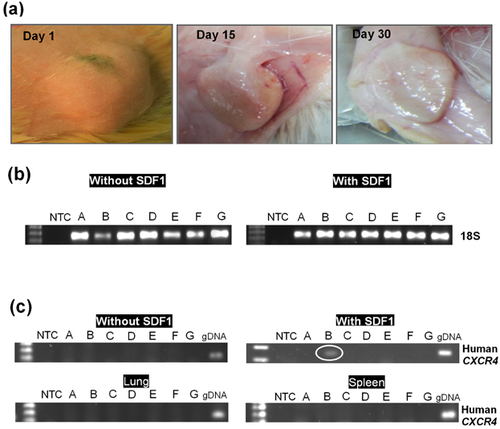
The CH-GP-HEC with final concentration of chitosan, GP, and HEC; 12 mg/mL, 120 mM, and 4 mg/mL, respectively, gelled after 20 min at 37°C, which is optimum for clinical purpose. The gelation temperature and time of CH-GP-HEC hydrogel are adjustable and depend on the concentration of GP. CH-GP-HEC-based hydrogel was nearly degraded after one month in vivo (Figure 4A). Cells or/and therapeutic factors like SDF-1α can simply mix with the CH-GP-HEC hydrogel and injected to desired sites.
CXCR4 expression and SDF-1α release increase homing and retention
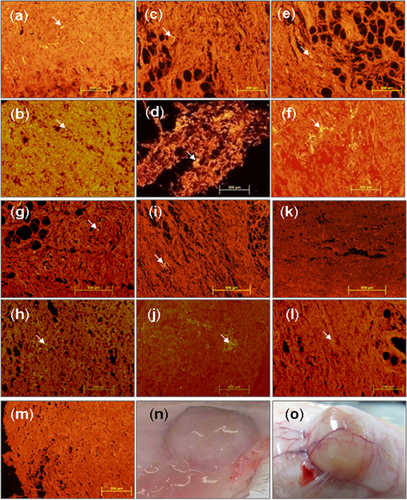
Although a few recent studies reported the in vivo SCs recruitment using SDF-1α-containing scaffold in a subcutaneous implantation model (Bladergroen et al., 2009; Kimura and Tabata, 2010; Thevenot et al., 2010; Chen et al., 2013; Zhang et al., 2013), no available studies investigated the recruitment and retention of pretreated ASCs in vivo. In this study, we designed a delivery platform for the controlled release of SDF-1α, an important chemokine for stem cell recruitment and homing, to investigate effect of pretreatment with DFX on ASCs and the efficiency of the three delivery routes. Results showed that pretreatment of hASCs with DFX can improve the efficacy and retention of hASCs mixed or migrated toward SDF-1α-releasing hydrogel injected subcutaneously in dorsum of Wistar rat in all three delivery routes (Figures 5A–5F) as compared with untreated experimental groups E, F, and G (Figures 5G–5L). On the other hand, the enriched population of DiI-labled cells was observed in the hydrogel containing SDF-1α as shown in Figures 5B, 5D, 5F, 5H, 5J, and 5L compared to the hydrogel without SDF-1α in Figures 5A, 5C, 5E, 5G, 5I, and 5K, respectively. For example, in delivery route 1 (mixed), incorporated SDF-1α effectively retained DFX-pretreated ASCs within the CH-GP-HEC hydrogel (Figure 5B). Additionally, since human specific genes were also detected by PCR only in experimental group B where DFX-pretreated ASCs were mixed with SDF-1α-containing hydrogel (Figure 4C), but not all other groups, it was concluded that SDF-1α operates in both homing and retention of CXCR4-positive cells. Furthermore, intensive cell infiltrations were observed within the CH-GP-HEC in this group accompanied with vascularization nearby (Figure 5O). This result was confirmed by the fact that the CH-GP-HEC hydrogels provided optimally permissive structural support for migration of cells (Figure 4B), indicating the hydrogels to be compatible with homing of endogenous cells.
Discussion
Various strategies to improve homing of MSCs have been reviewed recently by Naderi-Meshkin et al. (2014b). One approach to exploit efficient cell-based therapy is to transplant MSCs that overexpress CXCR4. Different manipulations have been undertaken to increase CXCR4 expression to allow efficient SDF-1α-induced stem/progenitor cell mobilization following a damage (Cheng et al., 2008; Zhang et al., 2008; Tang et al., 2009; Liu et al., 2013), thus mediating their homing and retention to the site of injury. Another approach is augmentation of SDF-1α at the site of injury through overexpressing or by a drug delivery system (Thevenot et al., 2010; Lau and Wang, 2011). Given that both overexpression of CXCR4 and sustained-release of SDF-1α improve the outcome individually, it would be expected that combining these two approaches would be even more effective. To this end, we have successfully implemented the use of preconditioning in order to overexpress CXCR4 in the transplanted ASCs and the CH-GP-HEC-based SDF-1α delivery system to direct the homing and retention of ASCs.
Our results showed that, in spite of low level existence of intracellular CXCR4 was shown by RT-PCR (Figure 3), no significant chemotactic responses to SDF-1α were observed in untreated ASCs (Supplementary Figure S2G). In contrast, after upregulation of intracellular CXCR4, which was provided by pretreatment of ASCs with DFX (Figure 3 and Supplementary Figure S2), the intracellular CXCR4 were functionally expressed on the cell membrane and mediated SDF-1α-dependent cell migration. Similarly, Kyriakou et al. (2008) reported that CXCR4 mobilization on the surface might need a threshold expression of intracellular CXCR4 transcript.
It is generally accepted that CXCR4 is the functional receptor corresponding to SDF-1α in regulating stem/progenitor cell migration (Sordi et al., 2005; Zaruba and Franz, 2010). However, controversial findings exist regarding the CXCR4 expression on the MSCs from different sources and species. Several previous studies reported the expression of CXCR4 on the cell surface of different kinds of MSCs (Honczarenko et al., 2006; Chamberlain et al., 2008; Baek et al., 2011). In contrast, our results demonstrated that CXCR4 expression in ASCs was predominantly intracellular, which confirmed several other reports (Wynn et al., 2004; Von Luttichau et al., 2005; Cao et al., 2013). Consistently, studies reported by others have shown that CXCR4 expression has a dynamic process and is regulated by sequestration (Wang et al., 2001), degradation via MMPs (Lapidot and Kollet, 2002; Kucia et al., 2005; Son et al., 2006), incorporation into lipid rafts (Wysoczynski et al., 2005), internalization (Dar et al., 2005; Croitoru-Lamoury et al., 2007; Ponte et al., 2007), post-translational and cell type-specific regulation mechanisms (Bruhl et al., 2003). Recently, it was shown by Beletkaia et al. (2014) that diffusion of CXCR4 in the plasma membrane under different conditions is dynamic and is not homogeneous. They found that the receptor is present in both mobile and immobile (∼20%) states and endocytosis inhibition results in an extra receptor fraction and confine diffusion. Previous studies have proposed additional reasons for contradictory results about expression of CXCR4, for example, intra and inter species heterogeneity of MSCs (Karp and Leng Teo, 2009; Naderi-Meshkin et al., 2014a), different extraction source of MSCs (Son et al., 2006; Ahmadian Kia et al., 2011; Baek et al., 2011), different passage number and evaluation methods of receptors (Wynn et al., 2004; Son et al., 2006; Ringe et al., 2007; Ahmadian Kia et al., 2011) or other variables such as isolation and processing techniques or other experimental conditions receptors (Chamberlain et al., 2008).
Overall, hydrogels proved to be promising biomaterials for injection into irregular cavities of lesions to recruit endogenous and exogenous progenitor cells to enhance tissue repair/regeneration (Chen et al., 2009; Kelm and Fussenegger, 2010). In our recently published study, it has been shown that the CH-GP-HEC hydrogel as a drug delivery system has initial burst release followed by a sustained release profile (Naderi-Meshkin et al., 2014a), and therefore was used in current study for delivery of SDF-1α. It has been reported that a scaffold with the putative gradient of SDF-1α formed during the first phase followed by a slow release is appropriate for cell recruitment and retention (Ji et al., 2013). In addition, several studies have demonstrated that the effective threshold of SDF-1α for cell recruitment is 5–40 ng/mL (De Falco et al., 2004; Ji et al., 2013), which is achievable by loading of 100 ng/mL SDF-1α into the CFH-GP-HEC injectable hydrogel, which has been used in our current study. Our results showed that SDF-1α plays the role in both chemoattraction and retention of ASCs, which is consistent with a previous report (Grunewald et al., 2006). Additionally, it was demonstrated that the combined delivery of ASCs and SDF-1α with CH-GP-HEC induces angiogenesis (Figure 5O), although further experiments are required in this context. Similarly, Schantz et al. (2007) have shown that the presence of native MSCs infiltrating into the scaffold was concomitant with angiogenesis and vascularization. In another study, Zaruba and Franz (2010) found that SDF-1α-induced VEGF secretion by cells profoundly modulate the angiogenic activity.
Conclusion
From three different delivery routes, the route in which the cells were mixed with CH-GP-HEC injectable hydrogel led to better results than systemic delivery and local injection. In this route, the cells were not trapped in the lung and spleen (which is the main limitation of systemic delivery) and also have not escaped from the site of injection (which is the limitation of local injection). In addition, it is concluded that the combined strategy with harnessing SDF1/CXCR4 axis encourages site-directed homing and increase retention of ASCs. Hence, SDF-1α as homing factors and cells retainer concomitant with preconditioning might be applied as a new way to achieve better therapeutic outcomes for cell therapy in the future.
Acknowledgments and funding
The authors thank Murk Bottema for his help in correcting the English. This work was financially supported by grants from the Iranian Council of Stem Cell Technology (project no. 3001), Ferdowsi University of Mashhad, and Iranian Academic Center for Education, Culture and Research (ACECR).
Conflicts of interest
The authors have no conflict of interest to declare.




