Personalized evolutionary hypothesis of breast neoplasm based on differential hybridization signals of subtelomeric probes
Abstract
The limited availability of data on somatic gene evolution in breast cancer is challenging item to apply an appropriate clinical management for each individual. As human subtelomeric sequences are diverse-prone and variable, they can be considered as hot spots for analysis of the health or disease status. We compared the hybridization signals of subtelomeric sequences in normal cells with those in auxiliary lymph nodes (ALNs) isolated from a single patient. Distinct signal intensities were found in all chromosomes: weak (5, 6, 9–12, and 16–19), medium (1, 5–9, 19, and X), and strong (2, 5, 9, 10, 16, and 18) intensities. Signal intensities in the patient's ALN and lymphocytes were higher than in normal tissues. The absence and presence of one or more hybridization signals and the presence of signals in the p and q arms were also variable. Whereas chromosomes 2, 3, 5, 7, 8, 10, 16, 18, 20, and X showed three hybridization spots, chromosomes 1, 4, 9, 12, 17, and 18–19 presented a reduced signal in the ALN and lymphocytes. In addition, signal intensity in the p arm was higher than in the q arm in most patients’ chromosomes. Therefore, we propose that subtelomeric hybridization be followed periodically in individuals with breast neoplasm to provide specific patterns. Such profiling could be considered as a prediction marker throughout the patients’ life. Together with the Ki67, cyclin D1, and cyclin E expression profiles, the subtelomeric hybridization profile could provide complementary information for cancer management.
Abbreviations
-
- ALN
-
- auxiliary lymph node
-
- BC
-
- breast cancer
-
- Chr
-
- chromosome
-
- FISH
-
- fluorescence in situ hybridization
-
- G
-
- genomic
-
- Q-FISH
-
- quantitative FISH
-
- SCN
-
- signal copy number
-
- SI
-
- signal intensity
-
- ST
-
- subtelomere
-
- TL
-
- telomere length
Introduction
Breast cancer (BC) is the most common cause of cancer mortality among women worldwide. Therefore, providing reliable cellular targets for early detection of biological behavior and an appropriate clinical management is urgent. In the early 1970s, it was initially recognized that chromosomes (Chrs) are not able to replicate their terminal regions. Leonard Hayflick stated a limited somatic cell division theory, in which DNA sequences could be lost during cell replication. A critical level would be reached, thus triggering cell division termination (Olovnikov, 1971, 1973).
The terminal region of Chrs is formed by telomere repeats followed by subtelomere (ST) sequences, which are characterized by repeats sequences (Churikov and Price, 2008). Therefore, length of telomeres and complexity of STs could indicate the evolutionary history of the cell (Denayrolles et al 1997; Van Overveld et al., 2000; Linardopoulou et al., 2001). It has been shown that reduced telomere length (TL) in peripheral blood lymphocytes is correlated with an increased risk of developing cancer (Pooley et al., 2010). We have also reported that diverse TL in cancer cells play a critical role in cancer progression. Furthermore, the genomic and somatic evolution, TL diversity, and heterogeneity in BC and brain tumor have been previously published (Mehdipour et al., 2011). Breast cells are in close contact with the lymphatic channels, which pass through lymph nodes, acting as a filtering system against hazard substrates and cancer cells. The number and nature of the auxiliary lymph node (ALN) are usually used as a supportive and diagnostic tool in BC management (Fregnani and Macea, 2009).
Subtelomeres have also been assayed by fluorescence in situ hybridization (FISH) probes in individuals with mental retardation (Flint et al., 1995; Knight et al., 1999). Later, quantitative-FISH analysis was performed on individual Chrs (Zheng et al., 2009, 2011). Subtelomeres corresponds to about 0.1% of the human genome, being involved in more than 40% of interchromosomal duplications (Linardopoulou et al., 2003). This region is prone to DNA breaks and repair (Fagagna et al., 2003; Rudd et al., 2007). However, the precise role of STs in cancer development and progression requires further investigation, especially in BC.
Due to the high diversity, distinct evolution, tumor heterogeneity, and aging of patients, the importance of major and minor ST differences in cells is relevant. Such differences might affect tumor behavior, especially in breast neoplasm, including benign tumors and BC. In BC, for example, a severe heterogeneity could be traced within a specific cancer type. Therefore, measuring the global TL length as a sole diagnostic criterium seems inadequate. In this sense, ST analysis by FISH could be a complementary information regarding cancer predisposition, prognosis, and prediction for further clinical management. In addition, it is worth remembering that telomere, telomerase, and STs act as a triangle and three-edged sword, by which decision making on patient's aging and cancer development would be possible.
We have recently reported a different profile of FISH signal, which reflects a distinct copy number of ST sequences of buccal and ALN cells and provides a personalized signature for the progress of breast neoplasm (Mehdipour et al., 2013). Given this personalized insight, the aim of the present study was to analyze the ST profiles for distribution of hybridization signals and intensity of individual p and q Chr arms in non-affected (genomic) and ALN cells of a female patient previously affected by primary proliferative ductal carcinoma. Moreover, the ALN was diagnosed as having a benign lesion, in which the ST characteristics could pave the way through a personalized follow-up study for the possible development of any malignant lesion in this patient.
Materials and methods
Patient information
A woman (44 years old) who was previously affected with infiltrating ductal carcinoma of left breast at age of 36 years, has gone under the excision of right breast mass measuring 8 cm in the greatest diameter. She was classified as a fibrocystic disease with moderate ductal hyperplasia and blunt duct adenosis, but negative for malignancy. The only removed ALN measured 4 cm in diameter. The histopathology results showed no evidence of malignancy. The patient provided a signed informed consent letter according to the requirements of the Tehran University of Medical Sciences.
Subtelomere multiprobe
The ST specific probe Chromoprobe Multiprobe (Cytocell) was used in multi-samples of a patient with primary BC. This system provides a unique condition for the application of multiple FISH probes to be hybridized with the whole set of Chrs in a separated status and at the same time and condition. Selection of probes was based on the most distal unique sequence of Chrs. These ST probes were capable to scan the ST enumeration and integrity (Cytocell). The multiprobe corresponded to a sequence located in the most distal region of Chr-specific DNA on each Chr. It was based on the 100–300 kb region of telomere-associated repeat. Ahead of this sequential segment, it contained the (TTAGGG)n sequence tandemly repeated with size in the range 3–20 kb. The original second-generation set of probes is derived from P1 artificial Chr (PAC) clones. The average probe size was 100 kb. The clones provided on the Chromoprobe Multiprobe-T System were mapped within a maximum size of the true telomere (600 kb). The p and q probes were conjugated with fluorescein isothiocyanate (FITC) and PE-Cy5 (Abcam), respectively, for the same Chr, and the signals were observed by using a fluorescence microscope (Leica Co., Germany).
Cell preparation
Cell populations included lymphocytes as genomic (G) cells, and ALN cells as neoplastic tissue. Lymphocytes were isolated by washing twice with phosphate-buffered saline (PBS), followed by fixation with methanol/glacial acetic acid (3:1). The ALN were obtained from paraffinized tissue samples (thickness: 5 μm) on positively charged slides. The slides were deparaffinized with xylene (5 min; twice), then rehydrated with 70% ethanol, and air-dried followed by pepsin treatment. The cells were washed twice in PBS and fixed in methanol.
Fluorescence in s itu hybridization (FISH)
The procedure was performed in the Chromoprobe Multiprobe-T (telomere) device from Cytocell. The set of specific clones were able to identify 41 of the 46 human telomeres (except the p-arm telomeres of acrocentric Chrs). Each of 24 multiprobe devices contained the specific probe for both the p and q arms of each Chr. The p- and q-arm probes were labeled with the fluorophores as recommended by the manufacturer.
Template slide spotting and slide pre-treatment were performed as recommended (Cytocell). The template slide was washed in 2× SSC (0.03 M NaCl, 0.3 M sodium citrate, pH 7). Slides were dehydrated through the 70–100% ethanol series and incubated with hybridization solution (1 μL) in the Multiprobe device. Denaturation was performed (75°C; 2 min) and followed by hybridization with the probes. Post hybridization washes were made with 0.4× SSC solution (72°C; 2 min) and in a 2× SSC/0.05% Tween 20 solution (30 s). The slides were then mounted and visualized. All Chrs (except the acrocentric D&G groups) were analyzed, and normal samples showed two green (p-arm) and two red (q-arm) signals per cell. Deletion of the ST regions led to the absence of signal. The visible SI was classified as weak, medium, or strong.
The Q-FISH was assayed using the Telomere PNA FISH Kit/Cy3 (code K5326; Dako, Denmark) according to the manufacturer instructions. Southern blot analysis was performed with the 12209136001 kit (Roche Diagnostics; GmbH, Germany).
Immunofluorescence
The immunofluorescence technique was used to detect protein expression at the cellular level (Mehdipour et al., 2011). Cells were stained with the FITC-conjugated monoclonal mouse anti-human Ki67 (isotype IgG1; Dako Cytomation), anti-cyclin D1 (IgG2a isotype; Biosource), and mouse anti-cyclin E (isotype IgG2b; Zymed Laboratories). In each sample, 1,000–5,000 cells were washed with PBS and incubated (4°C) with diluted antibodies. A secondary anti-mouse immunoglobulin G (IgG) was used when required. After mounting the slides, they were examined using a LEICA, DM RXA2-fluorescence microscope.
Data analyses
Data were analyzed with the SPSS statistic program (v. 17). The results are expressed as mean ± standard deviation (SD). Expectation-Maximization (EM) algorithms were used to achieve a reliable standard deviation (SD). This strategy was used to stabilize the available amount of each Chr. Different numbers of cells were analyzed for ALN (8490) and lymphocytes (40,197). One-way analysis of variance test used to compare the differences between frequencies of individual Chrs. The Pearson Correlation coefficient test was calculated to obtain the correlations between target factors. This procedure was authorized on the basis of Central Limit Theorem. Binary Logistic Regression was used to analyze the essential regression models. Significance was determined at P < 0.001 and P < 0.05.
Results
Signal copy numbers and signal intensities in ALN and G cells
Two cell populations, including normal and abnormal cells, were analyzed in order to assess their heterogeneity. The SCN and SI of FISH were used to characterize the STs present in each or all Chrs and distinguish the p and q arms.
Figure 1 shows, a typical labeling of ALN and relatively unaffected lymphocytes from the same patient, with specific probes for the p and q arms of Chr 1. Analysis with all probes showed that cells with positive signals correspond to 11.2% (4506/40.197) for ALN and 28.4% (2415/8494) for unaffected G cells. Highly significant differences were found in labeling each Chr (P < 0.001) when the status of the p/q ratio for SCNs in the p and q arms of ALN cells and lymphocytes was compared (Table 1). Moreover, distinct SIs, scored as weak-, medium-, and strong-intensity, also indicated significant mean differences in each Chr arm in signal-positive cells when comparing ALN and genomic cells (P < 0.001) (Table 2). However, lymphocytes without strong SI were found only in Chrs 6 and 12. A summary of differences between SCN and SI for the p and q arms of each Chr is shown in Table 3, emphasizing that the mean of ratio value for SCN and SI in genomic cells was significantly higher than in ALN cells. Specifically, the mean value for SI of p arms of all Chrs in G cells was also significantly higher than for weak and medium SIs in ALN cells, but not in cells with strong SI.
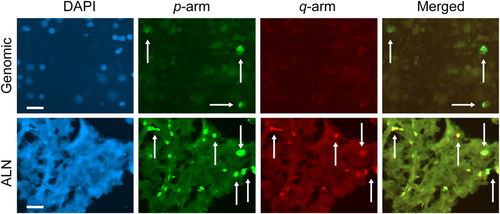
Subtelomeric signal copy number and fluorescence in situ hybridization signal intensity of the p and q arms of Chr 1 in a patient with breast cancer. Top panel shows genomic lymphocytes and the bottom panel shows auxiliary lymph node cells labeled with 4,6-diamidino-2-phenylindole (blue), p arm, q arm (fluorescein isothiocyanate), (PE-Cy5), and merged images. A small number of cells presented similar high values for signal copy number and signal intensity. The same pattern was found in different chromosome arms (not shown). White arrows indicate some cells labeled in the p and q arms. Bar size = 20 µm.
| SCNs per Chr | SIs per Chr | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Target tissues | Arms | Zero | One | Two | Three | Weak | Medium | Strong | |
| G>ALN | p | – | All Chrs, except 2, 6, 8, 11 | All Chrs | 3, 5, 6, 7, 10, 11, 18, X | All Chrs | All Chrs, except 6, 17 | All Chrs, except 6, 8, 11, 20 | |
| G>ALN | q | – | 1, 4, 5, 7, 9, 10, 11, 12, 13, 14, 17, 18, 19, 20 | 1–11, 13, 14, 18-22, X | 2, 3, 10, 20, 21, 22 | 1–10, 12–15, 18-22, X | 1–4, 7–15, 18–22 | 1–4, 7, 9–11, 13–15, 17–22, X | |
| ALN>G | p | All Chrs | – | – | 4, 8, 9, 16, 17 | – | All Chrs, except 6, 8, 11, 20 | – | |
| ALN>G | q | All Chrs, except 13, 16, 18 | – | 12, 15, 17 | - | 11, 16, 17 | 5, 6, 16, 17, X | 5, 6, 8, 12, 16 | |
| G = ALN | p | – | – | – | 20 | – | – | – | |
| G = ALN | q | – | – | – | – | – | – | – | |
- >, The value in G cells is greater than in ALN cells or vice versa; =, The values are equal in both G and ALN cells.
| SIs per Chr in the p arm | SIs per Chr in the q arm | |||||
|---|---|---|---|---|---|---|
| Target tissues | Weak | Medium | Strong | Weak | Medium | Strong |
| G>ALN | 2, 3, 5, 6, 9, 11, 12, 16, 17, 18, 19 | 1, 3, 5, 6, 7, 10, 11, 16, 18, 20, X | 2, 3, 7, 10, 12, 18, X | 1, 2, 3, 4, 6, 8, 9, 10, 14, 15, 20, 21, X | 1, 2, 3, 9, 10, 11, 12, 13, 22 | 1, 2, 3, 7, 9, 10, 11, 13, 15, 17, 19, 20, 22, X |
| ALN>G | 1, 4, 7, 8, 10, 20, X | 2, 4, 8, 9, 12, 17, 19 | 1, 4, 5, 6, 8, 9, 11, | 5, 7, 11, 12, 13, 16, 17, 18, 19, 22 | 4, 5, 6, 7, 8, 14, 15, 16, 17,18, 19,20, | 4, 5, 6, 8, 12, 14 |
| 16, 17, 19, 20 | 21, X | 16, 18, 21 | ||||
| G = ALN | – | – | – | – | – | – |
- >, The value in G cells is greater than in ALN cells or vice versa; =, The values are equal in both G and ALN cells.
| From total cells | From positive cells | |||||
|---|---|---|---|---|---|---|
| Chr-arms | Factors | ALN | G | ALN | G | P values |
| p | Zero | 0.90 ± 0.04 | 0.73 ± 0.13 | _ | _ | _ |
| One | 0.083 ± 0.031 | 0.11 ± 0.05 | _ | _ | _ | |
| Two | 0.012 ± 0.006 | 0.15 ± 0.09 | _ | _ | _ | |
| Three | 0.0017 ± 0.0018 | 0.0068 ± 0.0078 | _ | _ | _ | |
| Weak SI | 0.056 ± 0.025 | 0.16 ± 0.09 | 0.46 ± 0.13 | 0.48 ± 0.15 | ** | |
| Medium SI | 0.022 ± 0.011 | 0.067 ± 0.042 | 0.19 ± 0.09 | 0.19 ± 0.08 | ** | |
| Strong SI | 0.019 ± 0.007 | 0.041 ± 0.026 | 0.17 ± 0.07 | 0.13 ± 0.06 | ** | |
| q | Zero | 0.92 ± 0.18 | 0.88 ± 0.07 | _ | _ | _ |
| One | 0.042 ± 0.024 | 0.053 ± 0.030 | _ | _ | _ | |
| Two | 0.0031 ± 0.0028 | 0.065 ± 0.052 | _ | _ | _ | |
| Three | 0.0005 ± 0.0007 | 0.0016 ± 0.0042 | _ | _ | _ | |
| Weak SI | 0.022 ± 0.013 | 0.068 ± 0.047 | 0.23 ± 0.15 | 0.24 ± 0.19 | ** | |
| Medium SI | 0.013 ± 0.009 | 0.028 ± 0.022 | 0.15 ± 0.11 | 0.10 ± 0.10 | ** | |
| Strong SI | 0.0095 ± 0.0065 | 0.025 ± 0.019 | 0.11 ± 0.10 | 0.10 ± 0.09 | ** | |
- SI, signal intensity.
In cells with either one or two SCNs, the mean value in genomic cells was also higher than in ALN cells. In contrast, the mean values for three SCNs in genomic cells were higher than in ALN cells for half of the chromosomal set. As an example, we show the image of Chr 20 (Figure 2). For two and three SCNs, the mean value in genomic cells was higher than in ALN cells in each Chr.
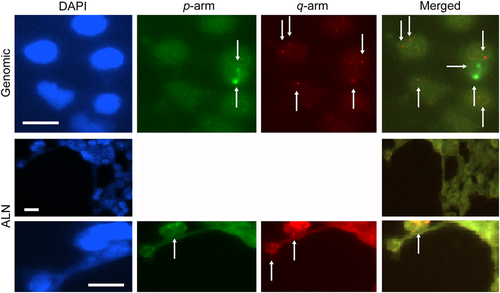
Subtelomeric signal copy number and signal intensity for the p and q arms of chromosome 20 in the genomic and auxiliary lymph node cells of a patient with breast cancer. Top row shows genomic cells and middle and bottom rows show auxiliary lymph node (ALN) cells labeled with 4,6-diamidino-2-phenylindole (blue); p arm, (fluorescein isothiocyanate); q arm (PE-Cy5), and merged images. Genomic cells show a remarkable signal with two low signal copy number (SCN) and signal intensity (SI) (see extra with arrows), and SCN is higher and SI is lower than in the ALN cells (see arrows in the q-arm labeling). The merged image shows a diverse characteristic for co-signaling of the p and q arms. The ALN cells in the p arm show lower SCN and SI than in the q arm, as also seen in the merged image. Bar size = 10 μm.
The mean difference between SCNs for the p/q ratio of ALN and genomic cells per individual Chrs from total cells was also evaluated (data not shown). In the case of labeling without SCN or presenting one signal, the mean value of the p/q ratio reflected significant differences for all Chrs (P < 0.001). Similar significance levels were obtained for two SCNs, except in Chrs 2, 11, 12, and 17, which showed a lower significance level (P < 0.05). In the cases of three SCNs, differences were highly significant for Chrs 3 and 20 (P < 0.001), and also significant for Chrs 2, 5, 7, 8, 10, 16, 18, and X (P < 0.05). For more information, the p/q ratio is also shown for all Chrs (Table 3).
Categorization of signal copy numbers in p and q arms
In most Chrs, the SI ratios obtained by probing p arm were lower than those obtained by probing the q arm, except for Chrs 2 and 18 in the ALN cells and Chr 4 in G cells. In some cases, absence of SCN or presence of one SCN explain the large differences between p/q ratios in Chrs, except for groups D and G due to lack of the p arm. In the case of one SCN for ALN cells and lymphocytes, the value in the p arm was higher than that in the q arm in all Chrs, except Chrs 3 and 4. In the case of two SCNs, the signals in the p arm were more intense than in the q- arm for all Chrs in the ALN (except in Chrs 2, 11, 16, and 5) and in the G cells (except in Chrs 4, 12, 16, and 17). In contrast, in the case of three SCNs, the most Chrs showed a greater labeling in the q arm. The mean differences between the p/q ratios for SCN and SI obtained by ST labeling of ALN and G cells are shown in Table 4. Interestingly, ALN cells that emitted no signal showed no significant correlation with other cells that emitted one, two, and three signals. Moreover, a negative correlation was found between cells that emitted no signal and those that emitted all SI degrees. Concerning G cells, highly significant correlations between different SCNs and SIs were also obtained for all Chrs.
| SCNs | SIs | ||||||
|---|---|---|---|---|---|---|---|
| No | One | Two | Three | Weak | Medium | Strong | |
| Mean difference between p/q ratios per individual chromosome | |||||||
| ALN | 0.85–0.960 | 1.09–12.00 | 1.66–5.00 | 1.32–11.33 | 1.03–11.33 | 1.41–20.00 | |
| Except: 8.5 (Chr 2); 1.02 (Chr 18) | 1.03–15.00 | Except: | Except: | Except: | Except: | Except: | |
| 0 (Chr 2,11,16); 0.45 (Chr 5) | 0.75 (Chr 3); 0 (Chr 1, 2, 4, 6, 9–12,17–20) | 0.85 (Chr 18) | 0.18 (Chr 3) | 0.39 (Chr 18); 0 (Chr 10) | |||
| G | 0.71–0.98 | 1.43–9.00 | 1.00–18.25 | 4.00 (Chr 2), 8.00 (Ch10); 0.50 (Chr 3, 20); | 1.00–26.00 | 1.06–8.00 | |
| Except: | Except: 0.75 (Chr 3); 0.9 (Chr 4) | Except: | 1.0 16.00 | Except: | Except: | ||
| 1.03 (Chr 4) | 0.50 (Chr 4); | Except: | 0.75 (Chr 4); | 0.65 (Chr 4); | |||
| 0.33 (Chr 19); | |||||||
| 0 (Chr 12,16,17) | 0 (Chr 1, 4–9, 11, 12, 17-19) | 0.87 (Chr 4); 0 (Chr 16) | 0 (Chr 6,16,17) | 0.27 (Chr 20); | |||
| 0 (Chr 6,12) | |||||||
| Mean p/q ratio for total SIs | |||||||
| ALN | 1.07 ± 1.65 | 2.53 ± 3.18 | 4.00 ± 4.05 | 2.01 ± 1.61 | 2.63 ± 2.58 | 3.00 ± 3.90 | 3.57 ± 5.21 |
| G | 0.64 ± 0.58 | 3.87 ± 7.88 | 5.48 ± 11.6 | 2.17 ± 3.09 | 5.55 ± 9.49 | 4.03 ± 5.94 | 2.28 ± 2.44 |
Telomere length analysis
In the present investigation, we evaluated the status of TL in lymphocytes and ALN cells. The ALN showed a decrease in TL as detected by Southern blot and Q-FISH analysis (Figure 3A).
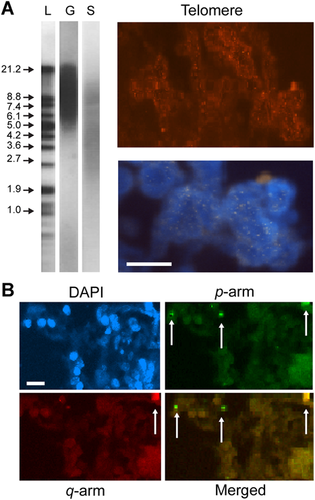
Comparison between the telomere length in genomic and somatic cells and subtelomeric profile in the auxiliary lymph node (ALN) of a patient with breast cancer. (A) The left panel shows a Southern blot of samples extracted from genomic (G) and somatic (S) samples and probed for telomere using non-radioactive chemiluminescent detection. Lane L corresponds to size markers (1 kb ladder) indicated in kDa. The right panels show cells labeled with quantitative fluorescence in situ hybridization probes for telomere (red) and the merged image labeled with 4,6-diamidino-2-phenylindole (DAPI). (B) ALN cells labeled with DAPI, subtelomeric probes in the p arm (fluorescein isothiocyanate), q arm cyclin PE-Cy5 and the merged images of p and q arms. The signal copy number (SCN) predominates for the p arm (see arrows). One cluster of cells showed co-stain, indicating a limited positive SCN and signal intensity. Bar size = 10 μm.
Protein expression of the key cell cycle markers
We also assayed the key targets involved in the cell cycle control of ALN cells to evaluate their degree of proliferation. In this regard, we analyzed the Ki67, cyclin D1, and cyclin E expression profiles (Figure 4) and we found a labeling diversity in the cell population.
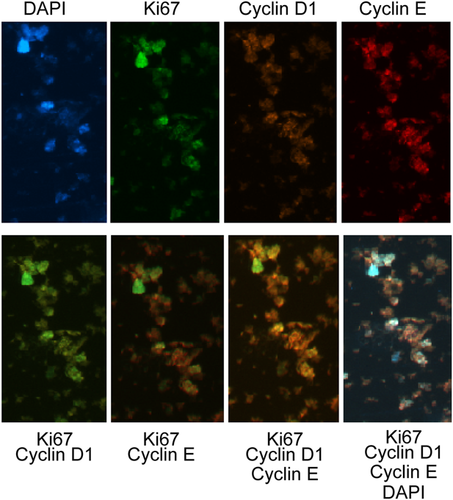
Expression of Ki67, cyclin D1, and cyclin E proteins in auxiliary lymph node (ALN) cells of a patient with breast cancer. The ALN cells were stained with 4,6-diamidino-2-phenylindole (blue) or anti-Ki67 (fluorescein isothiocyanate), anti-cyclin D1 R-PE, and cyclin PE-Cy5 (top images). At the bottom, the merged images indicate a similar expression for all markers, except Ki67, which showed low expression in most cells. Bar size = 10 µm.
Discussion
The human telomeric structure contains the simple tandem repeat (TTAGGG)n, which is accompanied by an extraordinary and complementary complex ST domain (Moyzis et al., 1988; Brown et al. 1990). In a previous publication (Mehdipour et al., 2011), we provided some insights comparing TL in tumor and other tissues. In addition, the ST of Chr 3q has been reported to be related to 35 other chromosomal ends (Trask, 2005; Brown et al., 1990), and the ST of Chr 7q was found to have a simple structure (Riethman et al., 1993). Eichler suggested that “exceptional duplicated regions underlie exceptional biology” (Eichler, 2001). The ST domain is a surprising region of the human genome, with an unusual evolutionary history, structure, and function. Therefore, STs are dynamic structures that may play a role in diversifying gene families, creating phenotypic diversity, and promoting disease-causing rearrangements.
However, no publication on human ST signals and SI for solid tumors, including BC, was available. Therefore, the aim of the present investigation was to explore the ST region by a systematic follow-up study of a patient with primary BC at the G and ALN levels. This study highlights the diversity and nature of ST signals and SI in the p and q Chrs’ arms of lymphocytes and ALN cells in a patient with BC.
Neoplastic tissues can act as proliferative reservoirs, supplying cells for cysts within the breast tissue or ALN. The formation of neoplastic tissue may be complicated by its progression. In this case, early detection, prediction, and prognosis is possible.
Regarding ST neighborhood, the telomeric diversity and evolution was discussed at the genomic and somatic levels (Mehdipour, 2013). The present results support the idea that remarkable cooperation between telomere and ST territories seems to exist, thus highlighting a further heterogeneity in human neoplasm. Although telomeres protect the Chr ends, SIs have extraordinary characteristics, being able to initiate and promote diversity.
Furthermore, there is a chain of events in the ST organization, including evolution, variation, and functional machinery, which may play a role in structural aberrations. STs are sensitive to macro- and micro-environmental factors, being prone to cause neoplastic initiation and progression.
Despite the limited publications on STs, this region is highlighted as “the gene-rich nature” of the ST region (Saccone et al., 1992). In addition, the role of ST rearrangements was reported in congenital abnormalities and mental retardation (Flint et al., 1995). Thus, here we tried to provide information to draw an individual map of signal distribution and intensity through the p and q arms of each Chr in different tissues of a patient with primary BC. This could provide a follow-up in different stages of the patient's life, allowing planning and management in the case patients with BC or even healthy individuals who have both been affected by hazard of environmental factors and inappropriate nutritional habits.
Collectively, higher and lower SCNs at ALN cells could be considered as a heterogeneous alteration at the neoplastic level; differently, lower SCNs in lymphocytes could be addressed as a reliable warning sign at the genomic level. Higher SCNs at the genomic level may suggest two possibilities: (i) an inherited positive prognostic trait or (ii) an acquired predisposing alteration.
Regarding the mean difference between SI values for the p arm, we found that all Chrs are predisposed to be affected by loss of genetic material (Table 1). Furthermore, the scenario in the q arm was found to be different regarding the mean difference between SCNs in the ALN and genomic cells. By focusing on cells without SCN, an exception was found in the q arm of Chrs 13, 16, and 18. By comparing the p and q arms for no signal, both arms of Chrs 1, 12, and 19 were involved.
However, more Chrs seem to have a higher mean value at the genomic level for the p arm. By comparing the p and q arms for the presence of three SCNs, Chrs 3 and 10 were found to be involved. By comparing the p and q arms for SI, all p arms showed higher mean values for weak SI in G cells on the basis of total cell count. But q-arm with weak SI was less frequent at the genomic level than in ALN, whereas Chrs 11, 16, and 17 showed lower SI value in the ALN than in G cells. However, reliable predisposing targets include Chrs 5, 6, 11, and X. Nevertheless, most Chrs are also trustable early detective targets at the genomic level (Tables 1 and 2). The use of the ST DNA probe only for Chr 17q was considered for breast tumor (Rashid-Kolvear et al., 2007).
Regarding the SI of the p arm, almost all Chrs showed higher intensity for somatic cells. In the ALN, a higher signal value was found in most Chrs, most characteristically in Chrs 6 and 8. In addition, weak SI at the genomic level was found only in Chrs 6 and 12. Collectively, the spectrum of diversity for SI at the genomic or ALN levels could reflect the ST heterogeneous behavior of the p and q arms in two different cells that show significantly different behavior. These findings could characterize different Chr arms with diverse behavior.
In addition, exploration of heterogeneity within a cell population with positive signals could suggest more reliable and specific estimations. Regarding the mean difference between different SI degrees in the p and q arms, we concluded that: (i) Chrs 2, 3, and 10 are modified in both arms, indicating that most Chrs behave differently in the p and q arms of G cells, even more than in ALN cells; (ii) in the ALN, most Chrs are differently affected by diverse SI categories, with values significantly higher than in G cells.
By evaluating both arms as a unique target, these findings show that there is no difference between the p and q arms. This is just to demonstrate that the p and q arms of individual Chrs with distinct regions and behaviors are influential targets in cancer cell biology. Such findings not only strengthen the analysis of individual Chrs, but also highlight the application of such a strategy in personalized cancer management.
By signal-positive cell-based analysis of the p arm in the whole Chr set for weak and medium intensity, the mean value for different SI categories in G cells was significantly higher than in ALN cells. Regarding q arm, only mean for cells with weak SI in lymphocytes were higher than in the ALN. Moreover, this also highlights that the p and q arms behave differently. However, these distinct results indicate the heterogeneous ST behavior at either genomic or ALN levels, and this could be considered as a predisposing factor at both genomic and neoplastic levels.
We also compared the global characteristics of SCN for the p and q arms as a common territory between ALN and G cells. For zero SCN, the mean in G cells was higher than in ALN cells and for all Chrs, except for Chrs 2 and 4, which may be involved in the pathogenic process at the somatic level. Regarding one SCN, no similar Chr involvement between two categories could be traced, including when the SCN in G cells was higher than in ALN and vice versa. This finding reflects the different behavior of G cells, which seems entirely different from that of ALN cells. However, the noticeable point is that losing genetic material is the key role in this category. Regarding two SCNs, the mean in G cells was higher than in ALN cells for all Chrs. This indicates the severity of the event in cancer cells, where down-regulated and heterogeneous ST alteration occurred at the neoplastic level with a benign histopathologic diagnosis. Such findings are warning signs for early management at the ALN level and probably at a further progressive level. Regarding three SCN, the mean in G cells was higher than in ALN cells. Interestingly, only Chr 7 showed similar mean values in the G and ALN cells. Finally, by comparing medium and strong intensities, it seems that Chrs 2, 3, 7, 10, and 13 were affected. Thus, the predisposition of both Chrs 2, 3, and 10 for different SI degrees in G cells is greater than in ALN cells.
The next step was to compare the total mean for SCN and SIs in the p and q arms in ALN and G cells as a Multi-Target Platform (Tables 1 and 2). It is worth to emphasize the importance of individual Chrs and each arm. With this regard, a comparison between the total mean for the p arm in ALN and G cells revealed a significantly higher value for one and three SCNs in G cells. However, such values were significantly higher for one and two SCNs of the q arm in the total cell count. These findings indicate that the p arm was significantly predisposed for signal loss and gain at the genomic level, highlighting the p arm as a reliable and prognostic marker candidate for early detection of neoplasm in this patient and probably in her relatives. However, the q arm was predisposed only for loss of the SCN.
Regarding different SI degrees, they were significantly higher in G cells than in ALN cells for both p and q arms (Tables 1 and 2). Such different intensities provide a wide spectrum for early qualification of both p and q arms, which also indicates molecular alterations at the ST region. In addition, the mean for SCN in ALN cells was higher than in genomic cells, indicating an evolutionary heterogeneity at the ALN level. By evaluating the total mean differences between SIs in the signal-positive cell population, a higher mean of weak and medium intensity was found for the p arm. However, weak intensity for the q arm was found only at the genomic level (Table 3). These results indicate that SI at the genomic level for the p and q arms is reliable.
Briefly, only a few cells exhibited a correlation between the high SCN and SI for the p and q arms of Chr 1 in both cellular environments. Furthermore, the heterogeneous distribution of SCN and SI of the p and q arms in both target tissues was remarkable. The strong SI in the q arm of Chr 1, even as a minor clone, could be a key element for a further developmental process, such as metastasis in ALN cells. In addition, the merged image of the p and q arms indicates the different ST characteristics of the p and q arms in G and ALN cells.
In addition, based on the comparative profile of the TLs, telomere signal pattern, and ST signal in the whole set of Chrs, we assumed that the global TL in the left breast of the same patient with primary infiltrating BC was initially low, either at the genomic or tumor level by the age of 36. Furthermore, telomerase activity was found to be positive in this patient (Mehdipour et al., 2011).
Most publications have focused on the molecular, and not on the classification of cellular patterns. The sequences of STs in Chrs 4p, 16p, and 22q were reported to have similar structures (Flint et al., 1997, 1997; Daniels et al., 2001). However, we have observed that these Chrs have different characteristics regarding SCN and SI. In this regard, it was previously stated that Chrs 20p, 4q, and 18p have a dual-domain structure (Chute et al., 1997). Our data allowed us to characterize individual Chrs according to the status of SCN and SI, which reflect reliable interactions in different cell populations and two different chromosomal arms. Moreover, interaction between frequent repeats leading to exchange has been reported only for Chrs 4q and 10q (Van Overveld et al., 2000). Such common characteristics could be specifically traced through different investigation channels, including SCN and SI.
Ratios for ST signals between ALN and genomic cells
The mean SCN of the p/q ratio for the total cell count, was significant for Chrs 2, 3, 5, 7, 8,10, 16, 18, 20, 21, and X (data not shown). In this regard, the absence or presence of different SCNs could indicate gain or loss of genetic material, being a possible key element in breast neoplastic biology. Given that the mean value for the p/q ratio in G cells is greater than in ALN cells, the involvement of common Chrs was found in cells with no signal, either one or two signals (Chrs 2 and 12), and three signals (Chrs 2 and 20). The most involved chromosomes in cells with one and two signals included Chrs 2, 5, 18, and 19. However, only Chr 2 was found in cells with three signals. Finally, Chr 2 revealed to be the common to Chrs involved in cells with two and three signals. Thus, the frequent Chrs most predisposed to harbor a variety of signals include number 2, followed by 12, 5, 18, and 19 as the most common ones.
Therefore, the p/q ratio for each Chr provides a new definition and clarification of the heterogeneity at the cellular level. Regarding different significant patterns for SCN in the p and q arm value, genomic cells tend to show remarkable p mean for three signals in Chrs 2 and 10. However, Chrs 3 and 20 showed a different value for the p/q ratio. In addition, the SI for the p arm was lower than that for the q arm only in Chrs 3 and 4, with intermediate SI in ALN and G cells, respectively. Regarding strong SI, only Chr 18 (in ALN cells) and 4, 19, and 20 (in G cells) showed a mean for the p/q ratio in the p arm, which was lower than that in the q arm. Such profile at the genomic level could be taken as an alarm for prediction and planning appropriate management through the patient's life.
Concerning the global mean difference between the p/q ratio values for SCN and SI, the ratio distribution for the SCNs shows a highly significant difference between the ALN and G cells. However, it was surprising that no specificity signal was observed when such data were compared with the value for each Chr (Table 4). Collectively, the p/q ratio indicates a highly significant difference between the two arms, and it is necessary to recognize them as two different and supportive regions.
As a complementary insight, the correlations between different categories of SCNs and SIs in G and ALN cells indicate different degrees of significant findings between these co-factors. Such correlations between ALN cells for all Chrs could highlight a harmonious cooperation between quality and quantity of these co-factors. Thus, SCNs showed a significant correlation with different degrees of SI in ALN cells, except between three SCNs and medium-intensity signal.
In summary, cells without SCN showed a negative correlation with other SCNs and SI categories at the genomic level. This indicates that the absence of ST signal and complete loss of the molecular reservoir in G cells may be taken as a reliable prognostic message for early detection. However, no correlation at the ALN level has a specific message, that is, these two co-factors act autonomously at the ALN level. Thus, each item has its own message. This process can be compared with a journey that begins at the genomic cells and proceeds harmoniously with the participation of the two ST co-factors, so that further action would be observed at the neoplastic level. These data can facilitate early predictive management to be performed. Cells with one ST signal have the same behavior at the G and ALN cells levels, showing a significant correlation between different categories of SCN and SI. This finding shows that G and ALN cells display only one SCN. Genomic cells with two SCNs showed a significant correlation with cells presenting one signal and different SI degrees. However, the negative correlation between the two-signal and no-signal categories, and the correlation between two SCNs and cells with three signals indicate that they behave independently. Moreover, it may be supposed that in this specific manner the results observed in lymphocytes could be also found in ALN cells. This is a reliable indication during further neoplastic disorder. A significant correlation with both co-factors was observed in G cells with three SCNs, but not in cells without signal. A significant correlation with other co-factors was observed in ALN cells, but not in cells without signal and medium-intensity signal.
TL analysis
Based on molecular and single-cell functional assays, a harmonious manner was confirmed by finding a low TL in tumor cells and week telomeric signals in G cells. The SCN and SI was also limited in ALN cells as well (Figure 3B). Thus, a down-regulated manner was confirmed within the triangle tissues in the patient with BC. Therefore, these data can be translated into cancer clinics.
Protein expression
Our results show that Ki67, cyclin D1, and cyclin E were expressed in the G and ALN cells. However, the benign nature of the ANL is apparent, as a limited number of cells presented high protein expression for Ki67 and cyclin E. This indicates the proliferative mode in the minority of the ALN cells, although expression of cyclins D1 and E was detectable. The significance of these findings and its relationship with malignancy should be further investigated.
Conclusion
Human subtelomeric sequences are variable, therefore we aimed to consider these regions as the hot spots marker to unmask the personalized evolutionary alterations in the breast neoplasm. The provided periodic charts allowed us to generate ST signal profiles characteristic of the alterations in SCN and SI in the patient's chromosomes (Figures 5 and 6). This resource can be also applied for other patients and establish correlations between evolution and the nature of BC and/or ALN lesions. The vertical and horizontal arrays show ST variations through individual chromosomes at the genomic and lymph node levels. Regarding the various categories of the p/q ratio for SCN, the most harmonic behavior was found in Chr 18, in its vertical profile. By focusing on the qualitative aspect, SI profiling mostly shows high intensity in almost all chromosomes, except numbers 1, 5, and 18. However, a non-uniformity in all SCN categories and both normal and medium SI was observed for Chr 18. In contrast, most characteristics for the absence of SCN and week SI show a regular mode. Our data, together with karyotyping, may lead to unmask the new fragile sites in breast neoplasm. The charts facilitate translation of the reliable personalized information, from lymphocytes to ALN or vice versa, for appropriate management. Furthermore, the periodic charts can be used as complementary single cell-based array systems in the cell domain, including tumor biology. Given the high significant correlation between the ST co-factors, stability and instability of the ST behavior (including quantities and qualities of genetic material), non-invasive samples (including genomic cells) are assayable and can be further followed up at the neoplastic level for each individual. In addition, analysis of ST profiling in the peripheral blood is a key gate for early prediction through bridging cancer cell biology to the personalized and translational insights. The cascade of evolution relies on tumor heterogeneity through the patients’ (and apparently healthy individuals’) life in different tissues, and these are the fundamental events of the provided Hypothesis, which facilitates an early detection within the ‘Prediction - Prevention - Prognosis’ frame.
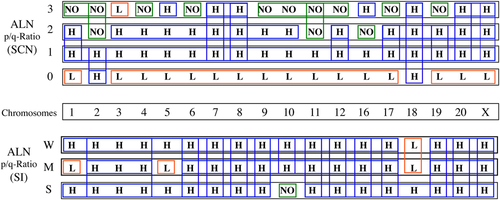
Chromosome profile of the p/q ratio for the subtelomere signal copy number (SCN) and signal intensity (SI) in cells of the auxiliary lymph node (ALN). The top chart shows the SCN labeling for each chromosome probe, where: H denotes p/q ratio < 1; L denotes p/q ratio > 1; and NO denotes cells without labeling when cells lose 1or 2 copies as estimated by labeled dots (normal situation) and cells with an additional copy recognized by the probe. The bottom chart shows a similar profile for SI, considering weak (W), medium (M) and strong (S) labeling. Colored boxes indicate a possible harmonization of data.
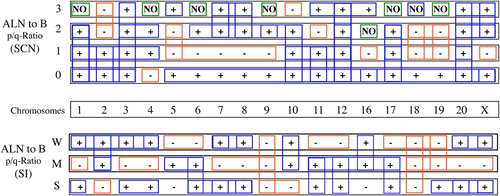
Chromosome profile of the p/q ratio for the subtelomere signal copy number (SCN) and signal intensity (SI) in the genomic (G) and auxiliary lymph node (ALN) cells. The top chart shows the enumeration of SCN for the p/q ratio of ALN as compared to blood cells (B). The bottom chart shows the SI for the p/q ratio of ALN as compared to blood cells (B) in the same conditions described in Figure 5. The symbols (+) and (−) indicate increased and decreased values, respectively. NO denotes that no change was observed. Colored boxes indicate a possible harmonization of data.
Acknowledgments
We thank Ms. Baghdasarian for her great assistance during the sampling process. We also thank the patient for her continuous cooperation through the sampling process and follow-up study.




