Roles of resolvins in the resolution of acute inflammation
Abstract
Resolution is an active process that terminates inflammatory response to maintain health. Acute inflammation and its timely resolution are important in host response to danger signals. Unresolved inflammation is associated with widely recurrent diseases. Resolvins, including the D and E series, are endogenous lipid mediators generated during the resolution phase of acute of inflammation from the ω-3 PUFAs, DHA, and EPA. They have anti-inflammatory and pro-resolving properties that have been determined in many inflammation studies in animal models. In this review, we provide an updated overview of biosynthesis, actions, and signaling pathways of resolvins, thereby underscoring their diverse protective roles and introducing novel therapeutic strategies for inflammation-associated diseases.
Abbreviations
-
- PMN
-
- polymorphonuclear leukocytes
-
- SPMs
-
- specialized pro-resolving mediators
-
- PUFA
-
- polyunsaturated fatty acids
-
- DHA
-
- docosahexaenoic acid
-
- EPA
-
- eicosapentaenoic acid
-
- LOX
-
- lipoxygenase
-
- COX-2
-
- cyclooxygenase-2
-
- GPCRs
-
- G-protein coupled receptors
-
- ChemR23
-
- chemerin receptor 23/chemokine receptor-like 1
-
- BLT1
-
- leukotriene B4 receptor
-
- ALX/FPR2
-
- lipoxin A4 receptor/formyl peptide receptor 2
-
- NF-κB
-
- nuclear factor kappa B
-
- PPARγ
-
- proliferator-activated receptor gamma
-
- PI3K
-
- phosphoinositide 3-kinase
-
- MAPK
-
- mitogen-activated protein kinase
-
- AKT
-
- protein kinase B
-
- mTOR
-
- mechanistic target of rapamycin
-
- JNK
-
- c-JUN N-terminal kinase
-
- ERK
-
- extracellular signal-regulated kinase
Introduction
Resolution of acute inflammation, or catabasis, leading to a return to homeostasis, is an ideal outcome of post-injury recovery (Bannenberg et al., 2005; Serhan and Savill, 2005; Serhan et al., 2008; Uddin and Levy, 2011). As an active rather than a passive process (Levy et al., 2001; Serhan, 2007; Stewart, 2009), resolution is characterized by orderly neutralization of noxious materials, non-phlogistic phagocytosis (termed efferocytosis) of apoptotic polymorphonuclear leukocytes (PMNs) by macrophages, and subsequent egress via lymphatics (Uller et al., 2006; Schwab et al., 2007). However, dysregulations in any part of these sequential events may cause failure of inflammation resolution and lead to fibrosis (Stewart, 2009). Failure of resolution underlies the mechanisms of a number of human inflammatory pathologies, such as obesity, asthma, cardiovascular diseases, neurodegenerative disorders, and cancer. Resolution is initiated by specialized pro-resolving mediators (SPMs) that are biosynthesized locally, such as the essential omega-3 (ω-3) polyunsaturated fatty acids (PUFA) derived lipid mediators (Serhan, 2004; Serhan et al., 2008). SPMs, generated during the resolution phase, inhibit PMN infiltration and enhance efferocytosis of apoptotic cells by macrophages. In man, they are synthesized transcellularly during the interaction between PMNs and endothelial/epithelial cells (Levy, 2010). Docosahexaenoic acid (DHA, C22:6n-3) and eicosapentaenoic acid (EPA, C20:5n-3), the main members of ω-3 PUFAs, exert protective and beneficial actions in many diseases (Simopoulos, 2002). In exploring the mechanisms of the recognized therapeutic values of ω-3 PUFAs, a new genus of endogenously lipid mediators, including resolvins, protectins, and maresins, have been identified. Resolvins, biosynthesized from precursor essential ω-3 PUFAs, EPA, and DHA, are more potent than DHA and EPA in their anti-inflammatory and pro-resolving actions (Krishnamoorthy et al., 2010; Isobe et al., 2013). Given their importance in inflammation resolution, resolvins represent a series of novel therapeutic agents for inflammatory diseases. We have summarized here the biosynthesis, intracellular functions, and effects of resolvins in inflammatory diseases.
The resolvin family
Biosynthesis of resolvins
Resolvins were recently identified molecules as being generated from ω-3 PUFA precursors and can orchestrate the timely resolution of inflammation (Figure 1). They are classified into D- and E-series; the resolvin D series (RvDs), including RvD1-RvD6, are derived from DHA, whereas the E-series, including RvE1-RvE3, are generated from EPA (Serhan, 2004; Serhan et al., 2004; Stewart, 2009; Spite et al., 2009 Spite and Serhan, 2010; Uddin and Levy, 2011; Recchiuti, 2013). DHA can also be converted to other bioactive compounds, such as Protectin D1 (PD1) and maresins (MaR1) (Hong et al., 2003; Serhan et al., 2004; 2006). Resolvins are biosynthesized through a lipoxygenase (LOX) mechanism or by the aspirin-triggered cyclo-oxygenase-2 (COX-2) pathway (Serhan et al., 2002; Uddin and Levy, 2011). Aspirin, after acetylation of COX-2, can shift its enzymatic activity from endoperoxidase to LOX-like, to trigger the endogenous biosynthesis of resolvins in an aspirin-triggered form (AT-Resolvins) (Uddin and Levy, 2011). The synthesis of RvDs requires a 2-step enzymatic process from DHA in vivo. Briefly, DHA is initially converted by 15-LOX in human or 12/15-LOX in mice to17S-hydroxy-DHA (17S-HDHA) and 17S-hydroperoxy DHA (17S-HpDHA), which are transformed to RvDs and PD1 via 5-LOX, respectively (Hong et al., 2003). In the presence of aspirin, acetylated COX-2 converts DHA into 17R-HDHA and 17R-HpDHA, which are transformed to 17R-RvDs (AT-RvDs) and 17R-PD1 by the oxygenation by 5-LOX (Serhan et al., 2002). Similarly, EPA uses enzymes, such as cytochrome p450, in microbes or aspirin-triggered acetylated COX-2 for the biosynthesis of 18R-hydroxyeicosapentaenoic acid (18R-HEPE) (Serhan et al., 2000; Arita et al., 2005a; 2005b). Aspirin treated COX-2 is also responsible for the production of 18S-HEPE (Oh et al., 2011). By interacting with 5-LOX, the intermediates 18R- and 18S-HEPE are converted to 18R- and 18S-RvEs, respectively, (Arita et al., 2005a; 2005b; Oh et al., 2011).
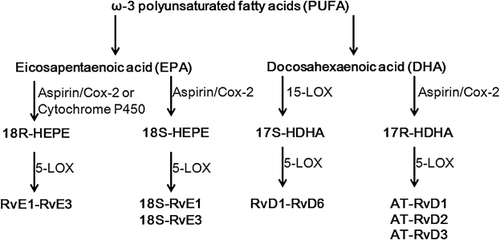
The biosynthetic enzymes have low expression levels or are inactive in normal tissues, but are activated during acute inflammation (Werz et al., 2002; Fukunaga et al., 2005; Ji et al., 2011; Stables and Gilroy, 2011). For example, COX-2 is nearly undetectable in normal tissues, but is rapidly induced at sites of inflammation (Fukunaga et al., 2005). 5-LOX is inactive in quiescent cells, but exhibits enzymatic function when triggered by intracellular calcium increase or phosphorylation (Werz et al., 2002; Stables and Gilroy, 2011). Pharmacological inhibition or genetic deletion of COX-2 or LOX blocks the timely resolution or leukocyte clearance (Fukunaga et al., 2005; Schwab et al., 2007). 15-LOX overexpression can inhibit inflammation in animal models (Serhan et al., 2003). EPA and DHA are not produced in human body, and it seems that exogenous EPA or DHA either from dietary consumption or by systemic administration can promote biosynthesis of resolvins. For example, RvE1 (Arita et al., 2005a), 18S isomer of RvE1 (Oh et al., 2011), RvE2 (Oh et al., 2012), RvD1 and RvD2 (Sun et al., 2007; Krishnamoorthy et al., 2010; Bento et al., 2011; Mas et al., 2012) are spontaneously produced in healthy human bodies taking aspirin, EPA, or DHA. Production of resolvins are increased in inflammatory diseases, such as high-severity multiple sclerosis (MS) (Pruss et al., 2013) and obesity (Titos et al., 2011), partially attributable to the upregulated expression of 12/15-LOX and 5-LOX (Titos et al., 2011). However, resolvins are rapidly deactivated by metabolism via enzymatic pathways (Seki et al., 2009), and deactivation is upregulated in disease conditions (Hong and Lu, 2013).
Receptors of resolvins
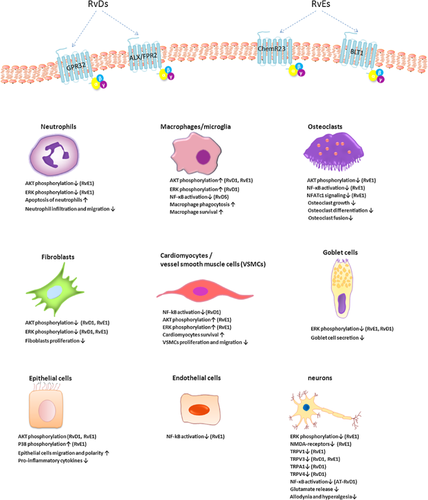
The biological actions of resolvins are predominately mediated by specific G-protein coupled receptors (GPCRs), which transduce intracellular signals in specific cell types resulting in pro-resolution and anti-inflammation (Figure 2, Table 1). Two receptors are involved in the actions of RvE1, namely chemokine receptor-like 1 (also known as chemerin receptor 23, ChemR23) and leukotriene B4 (LTB4) receptor (BLT1) (Wittamer et al., 2003; Arita et al., 2007). ChemR23 is a GPCR that also serves as a receptor for the chemotactic protein chemerin, encoded by CMKLR1 gene in humans (Bondue et al., 2012), whereas the mouse counterpart is termed Dez, with an 80% homology with human ChemR23 (Methner et al., 1997). ChemR23 is abundantly expressed on monocytes, macrophages, microglia, dendritic cells (DCs), natural killer cells (NK cells), dorsal root ganglion (DRG) neurons, platelets, vascular smooth muscle cells (VMSCs, and endothelial cells in humans and mice, but is weakly expressed in CD4+ T lymphocytes (Arita et al., 2005a; Campbell et al., 2010; Ishida et al., 2010; Haworth et al., 2011; Isobe et al., 2013). BLT1 is also a GPCR that is expressed on monocytes, neutrophils, eosinophils, and T cells (Yokomizo et al., 1997; Arita et al., 2007). RvE1 antagonizes the LTB4-BLT1 signaling by binding to BLT1 (Arita et al., 2007; Wan et al., 2011), whereas RvE2 partially interacts with BLT1, equipotent to RvE1. RvE2 is also a weak activator of the receptor ChemR23 (Oh et al., 2012). RvD1 is mediated by 2 GPCRs, human GPR32 and lipoxin A4 receptor/formyl peptide receptor2 (ALX/FPR2) (Krishnamoorthy et al., 2010). However, the murine counterpoint of human GPR32 needs to be identified. ALX is expressed predominantly on human epithelial cells and mouse neurons (Bonnans et al., 2006; Chiang et al., 2006; Pei et al., 2011). RvDs binding to GPR32 include RvD3, AT-RvD3, and RvD5 (Chiang et al., 2012; Dalli et al., 2013). AT-RvD1 is also the ligand for the receptor ALX (Bento et al., 2011). The specific receptors for RvD4 and RvD6 have still to be investigated.
| Mediator | Cell | Actions | Species | Receptors | References |
|---|---|---|---|---|---|
| RvE1 | Neutrophil | Inhibits neutrophil transmigration and infiltration to the inflamed sites | Mouse | ChemR23/ BLT1 | Arita et al., 2005a, 2007, 2005c; Bannenberg et al., 2005; Tjonahen et al., 2006; Campbell et al., 2007; Schwab et al., 2007; Jin et al., 2009; Tian et al., 2009; Li et al., 2010; Seki et al., 2010; Oh et al., 2011; Rajasagi et al., 2011; Schif-Zuck et al., 2011; Isobe et al., 2013, 2012 |
| Inhibits the superoxide release | Human | ChemR23/BLT1 | Hasturk et al., 2006 | ||
| increases CCR5 expression to terminate chemokine signaling | Human | Ariel et al., 2006 | |||
| Enhances neutrophil phagocytosis | Human | El Kebir et al., 2012 | |||
| Promotes neutrophil apoptosis | Human | El Kebir et al., 2012 | |||
| Eosinophil | Reduces eosinophil infiltration | Mouse | Aoki et al., 2010; Haworth et al., 2011 | ||
| PBMC | Attenuates LTB4-BLT1 induced calcium mobilization | Human | BLT1/ChemR23 | Arita et al., 2007 | |
| Monocyte | Limits monocyte chemotaxis by reducing L-selectin and CD18 expression | Human | Dona et al., 2008 | ||
| Macrophage | Inhibits the inflammatory macrophage infiltration | Human/mouse | ChemR23 | Jin et al., 2009; Fredman et al., 2011 | |
| Enhances macrophage phagocytosis | Human/mouse | Ohira et al., 2010; Fredman et al., 2011; Schif-Zuck et al., 2011; Takamiya et al., 2012 | |||
| Shifts the macrophage phenotype from M1 to M2 | Mouse | Navarro-Xavier et al., 2011 | |||
| Switches macrophages from CD11bhigh to CD11blow phenotype | Mouse | Schif-Zuck et al., 2011 | |||
| Enhances the emigration of apoptotic macrophages to lymphatics | Mouse | Schwab et al., 2007; Schif-Zuck et al., 2011 | |||
| Inhibits pro-inflammatory cytokines in macrophages | Mouse | ChemR23 | Ishida et al., 2010 | ||
| Microglia | Inhibits release of pro-inflammatory cytokines from microglia | Mouse | Xu et al., 2012 | ||
| Lymphocyte | Inhibits the infiltration of CD4+ T cells, and CD8+ T cells | Mouse | Li et al., 2010; Haworth et al., 2011; Rajasagi et al., 2011; Kim et al., 2012 | ||
| Promotes the apoptosis of TH-17 cells | Mouse | Haworth et al., 2008; Rajasagi et al., 2011 | |||
| Suppresses IFN-γ and IL-4 production in activated CD4+ T cells | Mouse | Kim et al., 2012 | |||
| NK cell | Increases cytotoxicity, migration of NK cells | Mouse | ChemR23 | Haworth et al., 2011 | |
| Increases the clearance of CD4+ T cells and eosinophils by NK cells | Mouse | ChemR23 | Haworth et al., 2011 | ||
| Platelet | Reduces ADP-stimulated platelet aggregation | Human | ChemR23 | Dona et al., 2008; Fredman et al., 2010 | |
| DC | Attenuates the chemotaxis of splenic DCs and BMDCs to lymph node | Mouse | Vassiliou et al., 2008; Isobe et al., 2013 | ||
| Suppresses TH-1 and TH-17 type cytokines splenic DCs and BMDCs | Mouse | ChemR23 | Haworth et al., 2008; Isobe et al., 2013; Miki et al., 2013 | ||
| Fibroblast | Reduces fibroblast proliferation | Mouse | ChemR23 | Qu et al., 2012 | |
| Goblet cell | Reduces LTD4-stimulated cell secretion in goblet cell culture | Rat | ChemR23 | Li et al., 2013 | |
| VSMC | Inhibits PDGF-stimulated VSMC chemotaxis | Human | ChemR23 | Ho et al., 2010 | |
| Epithelial cell | Attenuates inflammation by up-regulating ALPI on epithelial cells | Mouse | Campbell et al., 2010 | ||
| Promotes epithelium integrity by increasing epithelium migration | Human/mouse | BLT1 | Li et al., 2010; Zhang et al., 2010 | ||
| Endothelial cell | Dampens pro-inflammatory mediators in CRECs | Human | BLT1 | Tian et al., 2009 | |
| Osteoclast | Inhibits the osteoclast differentiation and growth | Mouse/Rabbit | ChemR23 | Hasturk et al., 2006; Herrera et al., 2008; Zhu et al., 2013 | |
| Inhibits osteoclast precursors maturation by blocking NFATc1 signaling | Mouse | Zhu et al., 2013 | |||
| Osteoblast | Up-regulates the OPG expression and activity of osteoblasts | Mouse | Gao et al., 2012 | ||
| Mast cell | Inhibits the infiltration of mast cells into the inflamed sites | Mouse | Kim et al., 2012 | ||
| Cardiomyocyte | Decreases apoptosis and increases viability of cardiomyocytes | Rat | Keyes et al., 2010 | ||
| 18S-RvE1 | Neutrophil | Limits neutrophil infiltration | Mouse | ChemR23/BLT1 | Oh et al., 2011 |
| Macrophage | Enhances macrophage phagocytosis and emigration to lymph nodes | Mouse | ChemR23/BLT1 | Oh et al., 2011 | |
| RvE2 | Neutrophil | Suppresses neutrophil infiltration by reducing CD18 expression | Human/Mouse | BLT1 | Tjonahen et al., 2006; Ogawa et al., 2009; Oh et al., 2012 |
| Macrophage | Enhances macrophage phagocytosis and up-regulates IL-10 level | Human | BLT1 | Oh et al., 2012; Isobe et al., 2012, 2013 | |
| RvE3 | Neutrophil | Stops neutrophil infiltration | Mouse | Isobe et al., 2012, 2013 | |
| Macrophage | Enhances macrophage phagocytosis | Mouse | Isobe et al., 2012, 2013 | ||
| 18S-RvE3 | Neutrophil | Limits neutrophil infiltration | Mouse | Isobe et al., 2012 | |
| Macrophage | Enhances macrophage phagocytosis | Mouse | Isobe et al., 2012 | ||
| RvD1 | Neutrophil | Reduces neutrophil infiltration | Mouse | ALX | Duffield et al., 2006; Jin et al., 2009; Murakami et al., 2011; Wang et al., 2011; Liao et al., 2012; Settimio et al., 2012; Tang et al., 2012 |
| Limits IL-8-induced neutrophil chemotaxis | Human | Kasuga et al., 2008 | |||
| Inhibits PMN transmigration across CREC barriers | Human | Tian et al., 2009 | |||
| Increases the phagocytosis of E.coli by human PMNs | Human | GPR32 | Chiang et al., 2012 | ||
| Reduces human neutrophil-endothelial interactions | Human | GPR32/ALX | Tian et al., 2009; Norling et al., 2012 | ||
| Reduces LTB4-stimulated actin polymerization | Human | GPR32/ALX | Krishnamoorthy et al., 2010 | ||
| Prevents neutrophil migration | Human | GPR32/ALX | Krishnamoorthy et al., 2010 | ||
| Monocyte | Limits IL-8 or LTB4-induced monocyte chemotaxis | Human | Kasuga et al., 2008; Jones et al., 2012 | ||
| Inhibits monocyte adhesion | Human/mouse | GPR32/ALX | Claria et al., 2012; Miyahara et al., 2013 | ||
| Inhibits the production of pro-inflammatory mediators in monocytes | Human | Hsiao et al., 2013 | |||
| Eosinophil | Reduces eosinophil infiltration | Mouse | Rogerio et al., 2012 | ||
| PBMC | Reduces the transcription of inflammatory genes in ALS PBMCs | Human | Liu et al., 2012 | ||
| Inhibits the secretion of inflammatory cytokines of PBMC from AD patients stimulated by FAM Aβ | Human | Mizwicki et al., 2013 | |||
| Macrophage | Limits inflammatory macrophage infiltration | Mouse | Duffield et al., 2006; Jin et al., 2009 | ||
| Enhances macrophage phagocytosis | Human/Mouse | GPR32/ALX | Krishnamoorthy et al., 2010; Chiang et al., 2012; Tang et al., 2012; Mizwicki et al., 2013 | ||
| Switches macrophages phenotype from M1 to M2 | Mouse | ALX | Hellmann et al., 2011; Titos et al., 2011; Hsiao et al., 2013; Zhang et al., 2013 | ||
| Switches macrophages from CD11bhigh to CD11blow phenotype | Mouse | Schif-Zuck et al., 2011 | |||
| Suppresses the pro-inflamamtory cytokines induced by aggregated SOD-1 | Human | Liu et al., 2012 | |||
| Enhances emigration of phagocytic macrophages to lymphatic system | Mouse | Schif-Zuck et al., 2011 | |||
| Prevents macrophage apoptosis by mediating oxidative burst | Mouse | Lee and Surh, 2013 | |||
| Microglia | Inhibits the expression of pro-inflammatory cytokines in microglia | Mouse | ALX | Xu et al., 2013 | |
| DC | Suppresses TH-1 cytokines release and MHC class II expression | Mouse | Miki et al., 2013 | ||
| Lymphocyte | Reduces the infiltration of CD4+ and CD8+ T cells | Rat | Settimio et al., 2012 | ||
| Augments human B cell differentiation to Ab-secreting phenotype | Human | Ramon et al., 2012 | |||
| Fibroblast | Inhibits fibroblast proliferation | Mouse | Qu et al., 2012 | ||
| Protects human gingival fibroblasts | Human | Mustafa et al., 2013 | |||
| Enhances proliferation and migration and bFGF release | Human | Khaled et al., 2013 | |||
| Inhibits pro-inflammatory mediators in fibroblasts | Human | Hsiao et al., 2013 | |||
| VSMC | Inhibits human VSMC proliferation, migration, superoxide generation and pro-inflammatory gene expression | Human | ALX/GPR32 | Miyahara et al., 2013; Zhang et al., 2013 | |
| Endothelial cell | Inhibits the pro-inflammatory cytokines from endothelial cells | Human | Merched et al., 2008; Tian et al., 2009 | ||
| Epithelial cell | Inhibits pro-inflammatory mediators in epithelial cells | Human | Hsiao et al., 2013; Dong et al., 2014 | ||
| Enhances migration and polarity of epithelial cells, reduces disruption of epithelial formation | Rat | ALX | Odusanwo et al., 2012 | ||
| Relieves epithelial permeability by protecting TJ protein | Mouse | Xie et al., 2013 | |||
| Osteoclast | Inhibits the osteoclast growth | Mouse | Yuan et al., 2010 | ||
| Podocyte | Protects podocytes by preventing loss of synaptopodin | Mouse | Zhang et al., 2013 | ||
| Mast cell | Attenuates the release of histamine from human lung mast cells | Human | Martin et al., 2012 | ||
| Goblet cell | Reduces LTD4-stimulated cell secretion in goblet cell culture | Human/rat | GPR32 | Li et al., 2013 | |
| AT-RvD1 | Neutrophil | Inhibits PMN infiltration | Mouse | ALX | Bento et al., 2011; Eickmeier et al., 2012 |
| Inhibits neutrophil-platelet interaction | Mouse | Eickmeier et al., 2012 | |||
| Macrophage | Increases macrophage phagocytosis | Mouse | Bento et al., 2011; Eickmeier et al., 2012; Rogerio et al., 2012 | ||
| Limits pro-inflammatory cytokines release | Mouse | Terrando et al., 2013 | |||
| Eosinophil | Reduces eosinophil infiltration | Mouse | Rogerio et al., 2012 | ||
| Epithelium | Improves epithelial barrier integrity | Mouse | Eickmeier et al., 2012 | ||
| Endothelium | Improves endothelial barrier integrity | Mouse | Eickmeier et al., 2012 | ||
| Platelet | Limits neutrophil-platelet interaction | Mouse | Eickmeier et al., 2012 | ||
| RvD2 | Neutrophil | Reduces neutrophil recruitment | Human/mouse | Duffield et al., 2006; Bento et al., 2011; Miyahara et al., 2013 | |
| Suppresses L-selectin shedding and CD18 expression | Human | Spite et al., 2009; Tian et al., 2009 | |||
| Regulates neutrophil-endothelial interaction | Human | Spite et al., 2009; Bohr et al., 2012 | |||
| Enhances phagocytosis of E.coli by human PMNs | Human | Spite et al., 2009 | |||
| Reduces neutrophil C5a mediated extracellular oxidative burst | Human | Spite et al., 2009 | |||
| Restores neutrophil motility | Mouse/Rat | Bohr et al., 2012; Kurihara et al., 2013 | |||
| Monocyte | Inhibits monocyte adhesion | Human/mouse | GPR32/ALX | Claria et al., 2012; Miyahara et al., 2013 | |
| Endothelium | Stimulates the production of vasoprotective and anti-thrombotic mediators | Human | Spite et al., 2009 | ||
| VSMC | Inhibits human VSMC proliferation, migration, superoxide generation and pro-inflammatory gene expression | Human | Miyahara et al., 2013 | ||
| Mast cell | Attenuates the release of histamine from human lung mast cells | Human | Martin et al., 2012 | ||
| RvD3 | Neutrophil | Inhibits neutrophil accumulation | Mouse | Duffield et al., 2006 | |
| Macrophage | Increases macrophage phagocytosis | Human | GPR32 | Dalli et al., 2013 | |
| AT-RvD3 | Neutrophil | Reduces neutrophil infiltration | Human/mouse | GPR32 | Dalli et al., 2013 |
| Macrophage | Increases phagocytosis by macrophages | Human | GPR32 | Dalli et al., 2013 | |
| RvD5 | Neutrophil | Increases phagocytosis of E.coli by PMNs | Human | GPR32 | Chiang et al., 2012 |
| Macrophage | Enhances phagocytosis by macrophages | Human | GPR32 | Chiang et al., 2012 |
Signal pathways in resolvin action
Nuclear factor kappa B (NF-κB)
Interfering with NF-κB signaling pathways has been postulated as a feasible therapeutic strategy for inflammatory diseases. Several lines of evidence indicate that resolvins inhibit pro-inflammatory mediators through modulating NF-κB pathway (Figure 2). Pretreatment with RvE1 inhibits NF-κB activity in ChemR23 (Arita et al., 2005a; Ishida et al., 2010) or BLT1-transfected cells (Arita et al., 2007). In murine osteoclast cultures, RvE1 binds to BLT1 and inhibits the activation of NF-κB, thereby interfering with osteoclast differentiation and bone resorption (Herrera et al., 2008). RvE1 (Campbell et al., 2010), together with RvD1 (Miyahara et al., 2013; Zhang et al., 2013; Dong et al., 2014; Erdinest et al., 2014) and RvD5 (Chiang et al., 2012) also attenuates the activation of NF-κB in human cells. RvE1(Ishida et al., 2010; Seki et al., 2010; Flesher et al., 2014), RvD1 (Wang et al., 2011; Liao et al., 2012), RvD2 (Bento et al., 2011) and AT-RvD1 (Bento et al., 2011; Eickmeier et al., 2013) mitigate the inflammation in murine pneumonia or colitis model by suppressing the activation of NF-κB in the inflamed tissues. RvD1-mediated attenuation of NF-κB signaling involves proliferator-activated receptor gamma (PPARγ) (Wang et al., 2011; Liao et al., 2012).
Phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways
Both PI3K and MAPK pathways are involved in resolvin-mediated resolution of acute inflammation (Rossi et al., 2006) (Figure 2). For example, RvE1 enhances the viability of RAW264.7 (Takamiya et al., 2012) and cardiomyocytes (Keyes et al., 2010), and promotes the migration of human epithelial cells (Zhang et al., 2010) by activating PI3K and/or MAPK. RvE1 suppresses murine osteoclast growth (Herrera et al., 2008) and enhances apoptosis of human neutrophils (El Kebir et al., 2012) by inhibiting protein kinase B (AKT) and/or extracellular signal regulated kinase (ERK) phosphorylation. Similarly, RvD1 promotes migration and polarity of salivary epithelium via the activation of PI3K/AKT pathway (Odusanwo et al., 2012), increases the phagocytosis of amyloid-β (Aβ) by macrophages in Alzheimer's patients through the activation of PI3K, protein kinase A (PKA), and MAPK kinase 1/2 (MEK1/2) (Mizwicki et al., 2013). Both RvE1 and RvD1 reduce the activity of AKT and ERK pathways, thereby preventing murine fibroblast proliferation (Qu et al., 2012). Activation of PI3K by RvE1 is initiated by RvE1-ChemR23 interaction, which results in phosphorylation of AKT and a ribosomal S6 protein via mTOR signaling (Ohira et al., 2010). In leptin receptor-deficient (db/db) mice, RvD1 enhances insulin-stimulated AKT phosphorylation in adipose tissue and the vasculature (Hellmann et al., 2011). By blocking ERK signaling, RvE1 abolishes transient receptor potential vanilloid subtype (TRPV1) and tumor necrosis factor-α (TNF-α)-evoked N-methyl-D-aspartic acid (NMDA) receptor hyperactivity in spinal dorsal horn neurons (Li et al. 2010; Xu et al., 2013). RvD1 and AT-RvD1 (Li et al., 2010) also prevent the release of inflammatory mediators from globlet cells via attenuating ERK activation. RvD1 also attenuates p38 activity in inflamed lung tissues (Wang et al., 2011).
MicroRNA
Resolvins regulate specific microRNAs (miRNAs) as part of their intracellular mechanisms (Bartel, 2009; Recchiuti, 2011; Krishnamoorthy et al., 2012; Recchiuti et al., 2013). For example, in human macrophages, RvD1 decreases miR-219, miR-21, and miR-146b (Fredman et al., 2012), but upregulates miR-146b in human macrophages overexpressing GPR32 (M-GPR32). In the zymosan-induced peritonitis model of ALX/FPR2 transgenic mice, RvD1 treatment upregulates miR-219 and miR-208a. RvD1 treatment downregulates miR-208a and upregulate 3 other miRNAs, miR-21, miR-146b, and miR-219, in wildtype littlermates (Krishnamoorthy et al., 2012). In human macrophages, RvE1 decreases miR-219 and miR-21, whereas RvD2 decreases miR-146b and miR-21 (Fredman et al., 2012). Given the involvement of miRNAs in inflammation (Sheedy et al., 2010; Recchiuti et al., 2011; Fredman et al., 2012), regulation of miRNA may be one of the mechanisms underlying the inflammation-resolution effect of resolvins.
Resolvins and resolution of inflammation
Unresolved inflammation is widely associated with recurrent diseases, such as atherosclerosis, diabetes, asthma, and neurological disorders. Resolvins therefore have potent multi-level mechanisms of action in diverse animal models (Table 2).
| Disease models/Species | Actions/Mediators | References |
|---|---|---|
| Colitis/mouse | Reduces mortality and body weight loss, inhibits PMN infiltration, down-regulates the production of pro-inflammatory mediators (RvE1, AT-RvD1, RvD2) | Arita et al., 2005c; Ishida et al., 2010; Bento et al., 2011 |
| Induces intestinal ALPI expression to mitigate the inflammation (RvE1) | Campbell et al., 2010 | |
| Periodontitis/Rabbit | Prevents tissue and bone loss, restores lost periodontal tissues (RvE1) | Hasturk et al., 2006; 2007 |
| TMJ inflammation/mouse | Prevents PMN infiltration into the inflamed sites (AT-RvD1) | Norling et al., 2011 |
| CNV/mouse | Mitigates IL-1β-induced CNV by reducing neutrophil and macrophage infiltration (RvD1, RvE1) | Jin et al., 2009 |
| EIU/rat | Ameliorates the eye damage by reducing the influx of neutrophils and T cells as well as suppressing pro-inflammatory mediators (RvD1) | Settimio et al., 2012 |
| Dry eye/mouse | Inhibits the infiltration of CD4+ T cells and neutrophils, suppresses the transformation from keratocytes to myofibroblasts (RvE1, RX-10001) | Li et al., 2010 |
| Stromal keratitis/mouse | Suppresses the influx of CD4+ T cells and neutrophis, increases IL-10 and decreases pro-inflammatory cytokines, thereby reduces angiogenesis and SK lesions (RvE1) | Rajasagi et al., 2011 |
| Atopic dermatitis/mouse | Reduces skin lesions by limiting leukocyte infiltration (RvE1) | Kim et al., 2012 |
| Contact dermatitis/mouse | Dampens TH-1 cytokines release and surface MHC class II expression in DCs (RvD1) | Miki et al., 2013 |
| Dorsal air pouch/mouse | Stops leukocyte infiltration (RvE1) | Arita et al., 2005a |
| Burn/mouse and rat | Prevents thrombosis of deep dermal vasculature, dermal necrosis and PMN mediated damage and improvesd survival (RvD2) | Bohr et al., 2013; Kurihara et al., 2013 |
| AKI/mouse | Attenuates functional and morphological kidney injury and reduces fibrosis (RvD1, RvDs) | Duffield et al., 2006 |
| UUO/mouse | Displays anti-fibrotic function (RvD1, RvE1) | Qu et al., 2012 |
| Nephropathy/mouse | Reduces proteinuria and interstitial fibrosis, shifts macrophages from M1 to M2 (RvD1) | Zhang et al., 2013 |
| Pneumonia/mouse | Decreases pro-inflammatory mediators, reduces neutrophil accumulation, enhances neutrophil apoptosis, improves survival rate and mitigates pneumonia (RvE1) | Seki et al., 2010; El Kebir et al., 2012 |
| Improves survival rate, decreases pro-inflammatory cytokines in BALFs, inhibits PMN recruitment (RvD1) | Wang et al., 2011; Liao et al., 2012; Wang et al., 2014 | |
| Relieves permeability edema (RvD1) | Xie et al., 2013 | |
| Improves epithelial and endothelial barrier integrity, decreases neutrophil influx and pro-inflammatory cytokines, increases epinephrine levels in BALFs (AT-RvD1) | Eickmeier et al., 2013 | |
| Asthma/mouse | Decreases eosinophil recruitment, activation, suppresses the levels of IL-5 and IL-17 and hyper-responsiveness (RvD1, AT-RvD1) | Rogerio et al., 2012 |
| Inhibits HLMC histamine release (RvD1, RvD2) | Martin et al., 2012 | |
| Promotes resolution by suppressing IL-23, IL-17 and IL-6 in BALFs and T cells (RvE1) | Haworth et al., 2008 | |
| Promotes resolution by increasing the clearance of eosinophils and T cells by NK cells (RvE1) | Haworth et al., 2011; Flesher et al., 2014 | |
| Prevents the development allergic airway responses (RvE1) | Aoki et al., 2008; 2010.; Haworth et al., 2008; 2011 | |
| Accelerates the resolution of lung inflammation, drives macrophage polarization from M1 to M2 phenotype (RvD1, AT-RvD1) | Hsiao et al., 2013 | |
| Obesity/mouse | Induces adiponectin secretion, reduces leptin, down-regulates inflammatory cytokines (RvD1, RvD2, AT-RvD1) | Claria et al., 2012 |
| Stimulates phologistic phagocytosis by macrophages from adipose tissue SVC (RvD1) | Titos et al., 2011 | |
| Diabetes/mouse | Improves glucose tolerance, insulin sensitivity and adiponectin production (RvD1) | Hellmann et al., 2011 |
| Decreases the apoptotic cells, accelerates wound closure (RvD1) | Tang et al., 2013 | |
| Elicits insulin-sensitizing effects (RvE1) | Gonzalez-Periz et al., 2009 | |
| Heart reperfusion/rat | Ameliorates reperfusion injury and limits the myocardial infarct size (RvE1) | Keyes et al., 2010 |
| Atherosclerosis/rabbit | Reduces cell proliferation, leukocyte influx and neointimal hyperplasis (RvD2) | Miyahara et al., 2013 |
| Peritonitis/mouse | Reduces total leukocyte and PMN infiltration as well as inflammatory mediators (RvE1, RvE2, RvE3, 18S-RvE1, 18S-RvE3, RvD1, RvD2, AT-RvD1, RvD3, AT-RvD3) | Arita et al., 2005a; 2007; Bannenberg et al., 2005; Tjonahen et al., 2006; Schwab et al., 2007; Spite et al., 2009; Stewart, 2009; Norling et al., 2011; 2012; Oh et al., 2011; Isobe et al., 2012; 2013; Dalli et al., 2013 |
| Enhances phagocytosis by macrophages (RvE1, RvE2, RvE3, 18S-RvE1, 18S-RvE3) | Arita et al., 2005a; 2007; Bannenberg et al., 2005; Tjonahen et al., 2006; Schwab et al., 2007; Oh et al., 2011; Isobe et al., 2012; 2013 | |
| Shifts macrophage phenotype switch (RvD1, RvE1) | Schif-Zuck et al., 2011 | |
| Enhances the emigration of phagocytes to lymph nodes and spleen (RvE1) | Schwab et al., 2007 | |
| E. coli infection/mouse | Reduces bacterial titres and prevents hypothermia (RvD1, RvD5) | Chiang et al., 2012 |
| Septic shock/mouse | Inhibits the release of septic mediators, and inflammatory cytokines, decreases peritoneal cell accumulation and the neutrophil population, reduces the hepatic apoptosis (RvD1) | Murakami et al., 2011 |
| Decreases bacterial levels, increases the phagocytosis by macrophages, enhances survival (RvD2) | Spite et al., 2009 | |
| Cognitive decline/mouse | Improves cognitive decline by abolishing synaptic dysfunction (AT-RvD1) | Terrando et al., 2013 |
| Pain/rat | Decreases mechanical allodynia (RvD1) | Quan-Xin et al., 2012 |
| Pain/mouse | Abrogates central sensitization, exerts strong anti-hyperalgesic actions (RvD1) | Huanget al., 2011 |
| Elicits anti-hyperalgesic effects (AT-RvD1) | Lima-Garcia et al., 2011; Xu and Ji, 2011 | |
| Alleviates inflammatory pain behaviors (RvD1, RvD2, RvE1) | Bang et al., 2010; Xu et al., 2010; Park et al., 2011 | |
| Inhibits spontaneous pain as well as heat and mechanical hypersensitivity (RvE1) | Xu et al., 2010 | |
| Reverses thermal hypersensitivity (AT-RvD1) | Bang et al., 2012 | |
| Renal transplantation/mouse | Prolongs renal allograft survival (RvE1) | Levy et al., 2011 |
Gastrointestinal disease
Crohn's disease and ulcerative colitis are the 2 major types of idiopathic inflammatory bowel disorders, characterized by strong leukocyte activation and infiltration into the intestinal tissues and release of pro-inflammatory cytokines and enzymes (Podolsky, 2002; Arita et al., 2005c). RvE1, AT-RvD1, and RvD2 mitigate inflammation in both dextran sulfate sodium (DSS)- and 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis, associated with reduced PMN infiltration and downregulation of interleukin-12 (IL-12), TNF-α, IL-1β, chemokine (C-X-C motif) ligand 2 (CXCL2), keratinocyte-derived chemokine (CXCL1/KC), and inducible NO synthetase (iNOs) in colonic tissues (Arita et al., 2005c; Ishida et al., 2010; Bento et al., 2011). AT-RvD1 and RvD2 block the PMN infiltration by suppressing the expression of the adhesion molecules, such as vascular cell adhesion molecule 1 (VCAM-1), intracellular adhesion molecule 1 (ICAM-1, also known as CD54), and lymphocyte function-associated antigen 1 (LFA-1) in DSS-induced colitis model (Bento et al., 2011). RvE1 also induces ALPI expression in intestinal epithelium by attenuating NF-κB activity, resulting in the resolution of inflammation (Campbell et al., 2010).
Oral disease
Periodontal disease is a bacterial infection in the bones and tissues supporting the teeth, characterized by neutrophil infiltration, impaired macrophage phagocytosis, osteoclast activation and osteoclast-mediated alveolar bone loss/resorption (Van Dyke and Serhan, 2003; Fredman et al., 2011). In P. gingivalis-induced rabbit periodontitis model, treatment with RvE1 prevents tissue and bone loss by interfering in osteoclast differentiation (Herrera et al., 2008), and the subsequent loss of osteoclasts by activation of BLT1 rather than ChemR23 (Hasturk et al., 2006). However, in mouse osteoblast culture, RvE1 has a direct bone-preserving function on osteoblasts via ChemR23 by uregulating osteoprotegerin (OPG) expression (Gao et al., 2013). RvE1 also prevents loss of periodontal tissues by reducing serum C-reactive protein (CRP) and IL-1β (Hasturk et al., 2007). Similarly, RvD1 also has an inhibitory effect of osteoclast growth (Yuan et al., 2010). Treatment with RvD1 abolishes TNF-α-induced disruption of rat salivary epithelial formation, enhances cell migration and polarity via ALX/FPR2, which may be beneficial to Sjögren's Syndrome (Odusanwo et al., 2012). In murine temporomandibular joint (TMJ) inflammation, the constructed AT-RvD1 enriched nanoparticles prevent PMN infiltration in inflamed sites (Norling et al., 2011). The pro-resolving effects of RvE1 and RvD1 have also been observed in inflamed human tissues and cells. For example, in localized aggressive periodontitis (LAP) patients, RvE1 effectively inhibits superoxide release by neutrophils (Hasturk et al., 2006), thereby mitigating bone destruction and rescuing phagocytic activity of macrophages (Fredman et al., 2011). RvD1 protects human periodontal ligament through enhancing periodontal ligament fibroblasts (PDL) proliferation, migration, basic FGF (bFGF) release, and subsequent wound closure (Mustafa et al., 2013). RvD1 also promotes human gingival fibroblasts (HGFs) survival by reducing the cytotoxicity of P. gingivalis-supernatant on HGFs (Khaled et al., 2013).
Ocular disease
Inflammation occurs in many eye diseases, such as age-related macular degeneration (AMD), uveitis, and conjunctivitis (Zhou et al., 2005; Chung et al., 2009; Zicari et al., 2013; Hu et al., 2014). Corneal and choroidal neovascularization (CNV) are associated with AMD, which involves neutrophil infiltration into the inflamed tissues (Grossniklaus et al., 2002; Cousins et al., 2004; Zhou et al., 2005). Treatment of murine CNV models with RvD1 or RvE1 reduces neutrophil and macrophage infiltration and the expression of TNF-α, IL-1α, IL-1β, vascular endothelial growth factor-A (VEGF-A), VEGF-C, and VEGFR2, resulting in attenuation of CNV (Jin et al., 2009). In rat endotoxin-induced uveitis (EIU) model, RvD1 markedly reduces influx of neutrophils as well as CD4+ and CD8+ T cells, and downregulates the expression of TNF-α, CXCL8 (also known as IL-8), and chemokine (C-C motif) ligand 5 (CCL5) in eye tissues via ALX/FPR2, thereby ameliorating eye damage (Settimio et al., 2012). Allergic conjunctivitis and early dry eye diseases are characterized by increased goblet cell mucin secretion into tears. RvD1 and RvE1 reduce leukotriene D4 (LTD4)-stimulated goblet cell mucin secretion in human and rat goblet cell culture via blocking LTD4-stimulated increase in intracellular calcium concentration ([Ca2+]i) and ERK activation (Li et al., 2013). In a murine model of dry eye, infiltrating CD4+ T cells and neutrophils are suppressed after treatment with RvE1 or its analog RX-10001. Both RvE1 and RX-10001 inhibit keratocyte transformation to myofibroblasts (Li et al., 2010). In the herpes simplex virus (HSV)-induced mouse stromal keratitis (SK) model, RvE1 significantly reduces angiogenesis and SK lesions by inhibiting the influx of CD4+ T cells and neutrophils, upregulating IL-10 and downregulating other pro-inflammatory cytokines (Rajasagi et al., 2011). Treatment of RvD1 or RvE1 in choroid-retinal endothelial cell line (CREC) or human PMNs inhibits PMN transmigration across CREC barriers and reduces various pro-inflammatory proteins (Tian et al., 2009). RvD1 (Erdinest et al., 2014) and RvE1 (Zhang et al., 2010) both enhance human corneal wound healing by reducing the level of pro-inflammatory cytokines or promoting epithelial growth factor receptor (EGFR)-mediated human corneal epithelial cells (HCECs) migration, respectively.
Skin disease
In 2,4-dinitrofluorobenzene (DNFB)-stimulated mouse atopic dermatitis model, RvE1 reduces skin lesions by lowering IL-4, interferon-γ (IFN-γ), and serum IgE, and suppressing the influx of eosinophils, mast cells, CD4+ T cells, and CD8+ T cells (Kim et al., 2012). RvE1 prevents leukocyte infiltration into TNF-α-induced dorsal air pouch (Arita et al., 2005a). In hapten-induced contact dermatitis model, phospholipase A2 group IID (PLA2G2D) has a pro-resolving function partly through RvD1, which dampens TH-1 cytokines release and surface MHC class II expression in lymph node cells or DCs (Miki et al., 2013). In mouse and rat burn models, RvD2 treatment prevents thrombosis of deep dermal vasculature, dermal necrosis and PMN mediated damage, thereby improving survival (Bohr et al., 2013; Kurihara et al., 2013).
Kidney disease
Acute kidney injury (AKI), usually resulting from ischemia/reperfusion injury, leads to chronic fibrosis and renal failure (Bonventre and Yang, 2011). RvDs, which are increased in the post-ischemic kidney, are important role in protection against AKI (Duffield et al., 2006). RvD1or RvDs (RvD1:RvD2:RvD3 = 1:2:1) given to mice before or at the onset of the ischemic injury markedly attenuates AKI and reduces fibrosis. This is associated with reduced accumulation and activation of inflammatory neutrophils and macrophages (Duffield et al., 2006). Both RvD1 and RvE1 possess direct anti-fibrotic action in a mouse model of unilateral ureteric obstruction (UUO) (Qu et al., 2012), possibly by reducing accumulation of myofibroblasts, deposition of collagen IV and myofibroblast proliferation (Qu et al., 2012). RvE1 also directly inhibits platelet derived growth factor (PDGF)-induced proliferation of primary mouse fibroblasts by ChemR23 (Qu et al., 2012). Podocyte damage or loss usually results in the development of glomerulosclerosis and renal failure. In a murine model of adriamycin (ADR) nephropathy, early treatment of RvD1 protects podocytes by preventing loss of synaptopodin, a marker of podocytes, by inhibiting phosphorylated synaptopodin/14-3-3β dissociation and switching macrophages from M1 to M2 phenotype, thereby attenuating proteinuria and interstitial fibrosis (Zhang et al., 2013).
Cardiovascular disease
RvE1 has direct protective effects on myocardial cells in vitro and in vivo. RvE1 given prior to heart ischemia/reperfusion ameliorates reperfusion injury and limits myocardial infarction size in rats (Keyes et al., 2010). RvE1 decreases hypoxia- or hypoxia/reoxygenation-induced apoptosis of cardiac embryonic myoblast cell line H9c2 partly through EGFR-mediated activation of PI3K/AKT, ERK, and inhibition of caspase-3 (Keyes et al., 2010). Atherosclerosis is a chronic inflammatory disease of the vascular wall, with the phenotype alterations of vascular endothelial cells (VECs) and vascular smooth muscle cells (VSMCs), as well as vascular injury. RvD1 downregulates expression of IL-8 and MCP-1 in human aortic endothelial cells (Merched et al., 2008) and reduces VECs permeability by protecting tight junction (TJ) proteins (Zhang et al., 2013). Both RvD1 and RvD2 dose-dependently inhibit human VSMC proliferation, migration, monocyte adhesion, superoxide generation, and pro-inflammatory gene expression (Miyahara et al., 2013). RvE1 inhibits PDGF-stimulated human VSMC chemotaxis by suppressing the activation of PDGF receptor-β (PDGFR-β) with no effect on VSMC proliferation and viability (Ho et al., 2010). Clinical studies show that resolvins protect vessels and cardiomyocytes in patients with abdominal aortic aneurysm (AAA) (Pillai et al., 2012). In a abbit artery injury model, RvD2 delivered locally reduces leukocyte recruitment and neointimal hyperplasia (Miyahara et al., 2013).
Metabolic syndromes
Obesity and type 2 diabetes have emerged as urgent metabolic inflammatory disorders. Adipose tissue expansion in obesity promotes accumulation of classically activated macrophages (M1 macrophages) that cause chronic inflammation, including insulin resistance, type 2 diabetes, dyslipidemia, cardiovascular disease, and non-alcoholic fatty liver disease (NAFLD) (Sanyal, 2002; Ferrante, 2007; Elks and Francis, 2010; Hellmann et al., 2011). In obese mice and leptin receptor-deficient (db/db) mice, treatment with RvD1 improves glucose tolerance, insulin sensitivity, and adiponectin production in adipose tissues (Hellmann et al., 2011; Claria et al., 2012) and accelerates wound closure (Tang et al., 2013). During this process, RvD1 reduces the formation of crown-like structures in inflammatory macrophages, shifts the M1/M2 balance by reducing CD11c and increasing macrophage galactose-type C-type lectin 1 (MGL-1) in macrophages (Hellmann et al., 2011), and stimulates the non-phlogistic phagocytosis by macrophages (Titos et al., 2011; Tang et al., 2013). AT-RvD1, RvD2, and RvE1 function like RvD1 (Gonzalez-Periz et al., 2009; Claria et al., 2012). RvE1 elicits significant insulin-sensitizing effects by enhancing adiponectin, GLUT-4, IRS-1, and PPARγ expression in adipose tissues and provides notable protection against obesity-induced insulin resistance and NAFLD in db/db mice (Gonzalez-Periz et al., 2009).
Airway inflammation/lung disease
Acute lung injury (ALI) is characterized by alveolar-capillary membrane disruption, extensive leukocyte infiltration, release of pro-inflammatory mediators, pulmonary edema and finally respiratory failure (Zhang et al., 2008; Yang et al., 2010). RvD1 (Wang et al., 2011; Liao et al., 2012; Xie et al., 2013; Wang et al., 2014), AT-RvD1 (Eickmeier et al., 2013), and RvE1 (Seki et al., 2010; El Kebir et al., 2012), each significantly decreases pro-inflammatory cytokines and chemokines, reduces neutrophil infiltration, improves survival rate and mitigates pneumonia in mice of ALI. RvD1 and AT-RvD1 attenuates neutrophil transmigration and filtration by improving epithelial and endothelial barrier integrity (Eickmeier et al., 2013; Xie et al., 2013). RvE1 also augments human neutrophil apoptosis by promoting phagocytosis (El Kebir et al., 2012). RvE1 level is upregulated in patients with cystic fibrosis (CF) lung disease, suggesting a role in improving lung function (Yang et al., 2012). Cigarette smoke induces oxidative stress, disables macrophage function, which contribute to ALI and chronic obstructive pulmonary disease (COPD). RvE1 treatment prior to cigarette smoke extract (CSE) suppresses superoxide production and cell death in macrophage cell line RAW264.7 and restores the phagocytic functions against E. coli (Takamiya et al., 2012). Similar inflammation resolution functions of RvD1 are seen in human lung fibroblasts, small airway epithelial cells (SAEC), and monocytes exposed to cigarette smoke, as well as in mouse models (Hsiao et al., 2013; Dong et al., 2014), regulating macrophage polarization from M1 to M2 phenotype by reducing iNOS expression and TNF-α, and increasing Arg1, Mrc1, and IL-10 expression (Hsiao et al., 2013). Asthma is an allergic airway inflammation, characterized by infiltration of eosinophils and T lymphocytes (Busse and Lemanske, 2001). RvD1, AT-RvD1, and RvE1 are potent pro-resolvers for asthma by preventing eosinophil and T lymphocyte recruitment, downregulating pro-inflammatory cytokines and enhancing clearance of allergen by macrophages in mouse model of asthma (Aoki et al., 2008; 2010; Haworth et al., 2008; 2011; Rogerio et al., 2012; Flesher et al., 2014). Ihibition of histamine release from human lung mast cells (HLMCs) could also be one of the anti-asthmatic mechanisms of RvD1 and RvD2 (Martin et al., 2012), and inhibition of NK cells may be responsible for RvE1-mediated resolution (Haworth et al., 2011).
Neurodegenerative disorders and inflammatory pain
Inflammation is also involved in neurodegenerative diseases, characterized by progressive loss of structure and functions of neurons. RvD1 improves macrophage-mediated phagocytosis of FAM-Aβ associated with Alzheimer's disease (AD) (Mizwicki et al., 2013), and suppresses pro-inflammatory cytokines in aggregated SOD-1 stimulated macrophages from Amyotrophic lateral sclerosis (ALS) patients (Liu et al., 2012). In high-severity MS patients, upregulation of RvD1 may be associated with disease progression (Pruss et al., 2013).
Tissue injury produces inflammatory pain in various conditions including arthritis, rheumatoid, etc. Pain is a perceptual process that arises from nocieption (detection of stimuli and the subsequent transmission of encoded information to the brain), ending with the symptoms hyperalgesia (response to noxious stimuli) or allodynia (response to normally innocuous stimuli) (Woolf and Salter, 2000; Kidd and Urban, 2001). Resolvins are potent analgesics for inflammatory pain. Intrathecal RvD1 administration abrogates central sensitization by reversing phosphorylation of NMDA receptors (NR1 and NR2B) in the spinal dorsal horn and significantly decreasing hyperalgesia and allodynia in TNBS-induced rat chronic pancreatitis (CP) (Huang et al., 2011; Quan-Xin et al., 2012). Systemic administration of AT-RvD1 causes marked anti-hyperalgesic effects in CFA-induced arthritis in rats by blocking TNF-α and IL-1β in the rat paw (Lima-Garcia et al., 2011; Xu and Ji, 2011; Bang et al., 2012). Intradermal pretreatment of RvD1 or intrathecal post-treatment with RvD1, RvD2 or RvE1 alleviates inflammatory pain in acute and persistent murine models of inflammation (Bang et al., 2010 Park et al., 2011; Xu et al., 2010). The molecular mechanism underlying the analgesic action of these mediators is partly attributable to the differential regulation of TRP ion channels (Park et al., 2011; Bang et al., 2012). The TRPs, which are temperature-sensitive, contribute to the genesis of inflammatory pain by both peripheral and central sensitization. RvD1 reduces inflammatory pain by inhibiting the activity of several TRP channels, such as TRP ankyryn 1 (TRPA1), TRPV3, and TRPV4, but not TRPV1 (Bang et al., 2010), and the anti-hypersensitive actions of AT-RvD1 may be attributable to the inhibition of TRPV3 (Bang et al., 2012). RvE1 abolishes TNF-α induced and TRPV1-mediated synaptic plasticity in the spinal cord (Park et al., 2011) and also significantly blocks TNF-α-induced NMDA receptor phosphorylation (Xu et al., 2010). Memory impairment and cognitive decline often occurs after the surgery. In a murine model of surgery-induced cognitive decline, AT-RvD1 suppressed superoxide production and TNF-α release in LPS-stimulated macrophages, and also improved cognitive decline by abolishing synaptic dysfunction (Terrando et al., 2013).
Resolvins – new drug candidates for inflammatory diseases
An ideal drug to treat inflammatory diseases would both suppress inflammation and activate resolution. However, many of the current anti-inflammatory drugs function simply by inhibiting mediators of inflammation. Some widely used anti-inflammatory therapeutics, such as inhibitors of COX-2 and prostaglandin E2 (PGE2), counteract pro-inflammation in certain settings without promoting pro-resolution (Serhan, 2004; Serhan et al., 2008). Although resolvins are produced in healthy human volunteers following the dietary supplement of DHA or EPA, they are deficient in certain diseases associated with chronic, unresolved inflammation. In specific tissues, supplementation with ω-3 PUFAs can potentially interfere with the formation of SPMs (Yin et al., 2009). Many studies in rodents and isolated human cells have unequivocally shown that resolvins have potent dual-acting roles with effective pharmacological activities, but rapid inactivation by local metabolic pathways (Arita et al., 2005c; Serhan, 2007; Haworth et al., 2008; Xu et al., 2010) and difficulty in producing from synthetic pathways (Ogawa et al., 2009) limit their clinical application. Hence, the development of more stable analogs of resolvins and improvement of delivery methods are required to enhance and prolong the pro-resolving actions of resolvins.
Resolvyx Pharmaceuticals (www.resolvyx.com) is the only company dedicated to drug development from resolvins. The company's product pipeline includes RX-10001 (resolvin E1) and RX-10045 (synthetic RvE1 analog) (Lee, 2012). RX-10045 has been successfully used in a phase II clinical study for the treatment of dry eye. This particular analog and clinical program has now been licensed to Celtic Therapeutics for further clinical development. RX-10001 is being evaluated clinically in many inflammatory diseases including dry eye, retinal diseases, cardiovascular diseases, asthma, and gastrointestinal diseases. Phase I studies of RX-10001 have been initiated to assess safety, pharmacodynamics and pharmacokinetics (www.clinicaltrials.gov). The resolution of acute-inflammation and resolvins are emphasized for the novel therapeutics.
Conclusions
Sustained inflammation and impaired resolution are major components of inflammatory disease pathophysiology. Resolvins, by binding to specific receptors, elicit multiple endogenous anti-inflammatory, pro-resolving, anti-fibrotic, anti-angiogenic, anti-infective, and anti-hyperalgesic actions. We have highlighted the biosynthesis and actions of resolvins in both rodents and human subjects. In-depth understanding of the protective roles and mechanisms may lead to the development of effective therapeutic approaches for many inflammatory diseases.
Acknowledgement and Funding
This work was supported by Shanghai Postdoctoral Scientific Program (12R21416100).
Conflict of interest
The authors declare no conflict of interest.




