Bone formation in adipose-derived stem cells isolated from elderly patients with osteoporosis: a preliminary study
Abstract
We have explored the osteogenic potency of adipose-derived stem cells from osteoporotic patients (opASCs). opASCs were osteogenically induced in vitro with collagen I hydrogel or in culture plate. Detection of alkaline phosphatase (ALPase) and cell mineralisation, and quantitative RT-PCR of collagen I, osteocalcin and bone sialoprotein were undertaken. Proliferation and morphology studies were also performed. After 14 days, opASCs-collagen I hydrogel composite was implanted into nude mice for 4 weeks prior to radiographic and histological analysis. Staining of ALPase and cell mineralisation was strongly positive in opASCs. Fibroblast-like opASCs induced with collagen I hydrogel were evenly distributed and proliferated at a higher rate than in culture plates, showing similar growth curves for both genders. Expression of ALPase activity, cell mineralisation and osteogenic specific genes were higher in opASCs with collagen I hydrogel (male samples had better osteogenicity than female samples) than in culture plates. After implantation for 4 weeks, radiopaque area signifying new bone tissue was observed in opASCs-collagen I hydrogel composite, with no donor gender differences. Thus opASCs with collagen I hydrogel have adequate osteogenic potency and offer new possibilities for osteoporosis-related bone tissue engineering in male and female patients.
Introduction
Osteoporosis, characterised by reduced bone mineral density and deteriorating bone microarchitecture, has become an increasing major public health concern as a result of the ageing population in developing and developed countries. Minor events, such as a fall from standing height, can cause severe fractures (commonly at the spine, hip, distal radius and proximal humerus) due to the profound degree of bone fragility in osteoporotic patients. The incidence of fracture varies with ethnicity, gender, age and height of the populations under study (Zhao et al., 2008; Couris et al., 2012). As the elderly population continues to increase, greater efforts will be required to improve quality of life and prolong life expectancy.
The treatment of osteoporotic fractures is made difficult by reduced callus formation and impaired biomechanical properties of bone tissue associated with a high risk of internal or external fixation failure. These problems are exacerbated when reconstructing large bone defects in patients who have suffered major trauma or have undergone tumour resection. Autologous bone grafting remains the therapeutic gold standard to reinforce bone mass and strength. However, limited graft availability, donor site morbidity and decreased bone marrow osteogenesis together limit its use (Hao et al., 2008).
The isolation of mesenchymal stem cells (MSCs) from bone marrow (BMSCs) has paved the way for cell-based bone tissue engineering as a promising alternative. Recovering MSCs from patients using minimally invasive surgical techniques enables cell number and differentiation potency to be manipulated in vitro according to the specific therapeutic need. A hybrid MSC scaffold with or without cytokines can then be transplanted to the bone defect site, enhancing the formation of new bone tissue. Adipose-derived MSCs (ASCs) possess similar multilineage differentiation capacity to BMSCs, but are easier to obtain in larger numbers, and are associated with relatively lower donor site morbidity (Hao et al., 2008). Our previous studies have demonstrated that ASCs possess good osteogenic potency at ectopic and orthotopic sites (Hao et al., 2008, 2010).
In osteoporotic patients, bone marrow adiposity decreases the osteogenic potential of BMSCs (Dalle Carbonare et al., 2009). We have isolated ASCs from subcutaneous adipose tissue of osteoporotic patients with hip fractures (intertrochanteric or femoral neck) who underwent osteosynthesis by internal fixation (Gama nail or dynamic hip screws) or total hip arthroplasty. The degree of osteogenesis has been estimated in vitro and in vivo after osteogenic induction with a collagen I hydrogel cell carrier.
Materials and methods
Ethics statement
The protocols were approved by Yantai YuHuangDing Hospital Medical Ethics Committee of Qingdao University Medical College (Yantai, China) (Approval ID: YYYLLS[2013]88). Written informed consent was obtained from the donor for use of this sample in research.
Isolation of adipose-derived stem cells from osteoporotic patients (opASCs)
The study population included 15 elderly patients (nine women and six men) with a mean age of 62.3 ± 13.4 years. The patients had a confirmed diagnosis of osteoporotic hip fragility fractures based on radiographic evidence and mechanism of injury. In addition, all patients fulfilled the World Health Organization (WHO) criteria for osteoporosis.
Subcutaneous adipose tissue from the hip was obtained during surgery. It was washed with sterilised phosphate buffered saline (PBS), finely minced, and digested by 0.1% collagenase I (Sigma, St. Louis, MO, USA) at 37°C for 1 h. Collagenase I was neutralised by adding an equal volume of control medium containing Dulbecco's modified Eagle's medium (DMEM; GibcoBRL, Carlsbad, CA, USA) and 10% fetal bovine serum (FBS; Sijiqing, Hangzhou, China). The tissue was filtered through a 150 µm mesh filter to remove the debris, and the filtrate centrifuged at 1,000 rpm for 6 min so that the pellet could be suspended in control medium. This suspension was plated onto conventional plates and cultured at 37°C in air plus 5% CO2. The culture medium was changed every 3 days. After reaching confluence, the cells were passaged with 0.25% trypsin/EDTA and replated at a 1:3 dilution.
Osteogenic differentiation and combination with collagen I hydrogel
Passage 3 opASCs used in our experiments were induced using osteogenic differentiation medium containing control medium plus 0.1 µM dexamethasone, 50 µg/mL ascorbic acid-2-phosphate 10 mM β-glycero-phosphate (all from Sigma). The medium was changed every 3 days.
Alkaline phosphatase (ALPase) activity (Gomori method) and calcium deposition (von Kossa staining) assays were used to measure the osteogenic potency of opASCs after 14 and 21 days, respectively.
Collagen I hydrogel was prepared as follows. Briefly, sterilised type I collagen powder (calf skin; Sigma) was processed into a 5 mg/mL solution by the addition of sterile acetic acid (0.1% v/v), mixed with 10 × DMEM and FBS at a volume ratio of 10:1:1. The resulting collagen solution was neutralised with sterile NaOH (1 N) to adjust the pH to 7.4. The solution was maintained at 4°C to prevent gel formation. Passage 3 opASCs were collected and resuspended in 100 µL collagen I hydrogel at 2 × 105cell/mL on ice. Gel formation occurred after exposure for 30 min at 37°C. The opASCs-collagen I hydrogel composites were cultured in osteogenic medium at 37°C and 5% CO2 in air for 14 days prior to in vivo implantation.
The DNA content of the cells was determined by fluorometric assay. On days 1, 7 and 14, the opASCs-collagen I hydrogel composite was dissolved by 2% collagenase I at 37°C for 45 min, neutralised in culture medium and centrifuged to form cell pellets, which were lysed by sonification. The homogenates were mixed with Hoechst 33258 stain (Doindo Lab., Japan). Emission and excitation spectra were obtained using a Modulus Microplate Luminometer (Turner BioSystems, USA) at 458 and 356 nm, respectively.
Controls were obtained by culturing 100 µL samples of opASCs from passage 3 that had been resuspended in osteogenic medium at 2 × 105 cell/mL.
ALPase activity, cell mineralisation under osteogenic differentiation
On days 7 and 14, cell pellets were prepared and lysed as described above. ALPase activity of lysates was measured by the release of p-nitrophenol from p-nitrophenyl phosphate (SIGMAFAST™, Sigma).
To evaluate cell mineralisation, cell pellets collected on days 7 and 14 were incubated overnight with 1 mL of 0.5 N HCl with gentle shaking. The Ca2+ ions were quantified using the o-cresolphthalein complex 1 method with a Calcium C kit (Wako Pure Chemical Industries, Osaka, Japan).
Quantitative real-time PCR of osteogenic specific genes
- COL (Forward: 5′-GCAAGGGAGAAAAGGGTGAACC-3′,
- Reverse: 5′-GTGGCTCCAGCAGGACCAG-3′),
- OC (Forward: 5′-CTCCAGGCACCCTTCTTTCC-3′,
- Reverse: 5′-ATTCCTCTTCTGGAGTTTATTTGGG-3′),
- BSP (Forward: 5′-ATACCATCTCACACCAGTTAGAATG-3′,
- Reverse: 5′-AACAGCGTAAAAGTGTTCCTATTTC-3′),
- β-actin (internal control) (Forward: 5′-ACAGAGCCTCGCCTTTGCC-3′,
- Reverse: 5′-ACATGCCGGAGCCGTTGTC-3′).
On days 1, 7 and 14, the total RNA content of opASCs in hydrogel or in control plates was evaluated using Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and were reverse transcribed to cDNA. Real-time PCR assay volume (25 µL) containing 1 µL cDNA, 10 µM gene-specific primers, 2× SYBR Premix Ex Taq™ (TaKaRa Biotechnology, Dalian, China), 50× ROX Reference Dye, and dH2O was incubated in an ABI 7500 Real-Time Thermocycler (Applied Biosystems, Foster City, CA, USA). After initial denaturation at 94°C for 10 min, the cells were subjected to 45 cycles at 94°C for 40 s, 60°C for 30 s and 72°C for 30 s. The levels of target genes were calculated using the comparative threshold-cycle (Ct) method.
Morphological observations
opASCs-collagen I hydrogel composite was examined by phase contrast microscopy 14 days after in vitro culture in osteogenic medium. It was fixed with 2.5% glutaraldehyde, dehydrated through a graded series of ethanol concentrations, critical-point dried and sputter-coated with gold on aluminium stubs for visualisation by scanning electron microscopy (SEM) (Hitachi High-Technologies Corp., Tokyo, Japan).
Ectopic bone formation
After 14 days of osteogenic culture the opASCs-collagen I hydrogel composites were transplanted into the space between the subcutaneous tissue and deep fascia of nude mice. Acellular collagen I hydrogel was implanted as a control. The animals were killed 4 weeks later and radiographed. The implants were removed and processed for histological analyses. Recovered constructs were fixed in 10% formalin neutral buffer solution, treated with decalcifying solution (10% HCl and 0.1% EDTA), dehydrated through a series of graded ethanol concentrations, infiltrated and embedded into paraffin wax. The samples were cut into 7 µm sections and stained with hematoxylin and eosin (HE).
Statistical analysis
SPSS Statistics 13.0 software (SPSS, Inc. Chicago, IL, USA) was used for the data analysis. Quantitative data were expressed as means and standard deviations (± SD). Student's t-test was used to analyze the results of DNA content, ALPase activity, extracellular mineralisation and the osteogenic specific genes expression level. P < 0.05 was considered statistically significant.
Results
In vitro culture of opASCs in osteogenic medium
At 14 days after osteogenic differentiation, opASCs expressed high ALPase activity (Figure 1a), seen as an extensively deep grey-stained area (Figure 1b). Marked calcium deposition in the extracellular matrix was seen in the form of different size black nodules (Figure 1c). Between days 7 and 14 the DNA content of opASCs-collagen I hydrogel increased at a significantly faster rate than in control opASCs (P < 0.05; Figure 1d). No gender differences were observed in opASCs-collagen I hydrogel composites (Figure 1e).
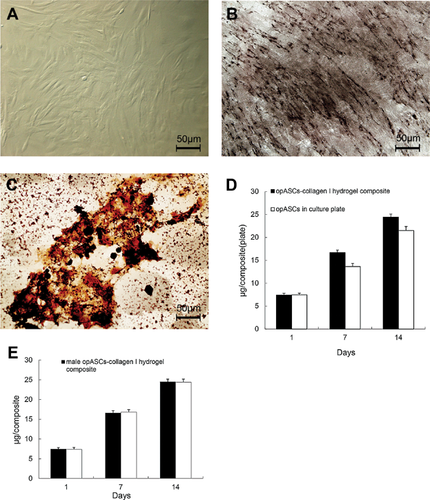
ALPase activity and extracellular mineralisation of opASCs-collagen I hydrogel composite
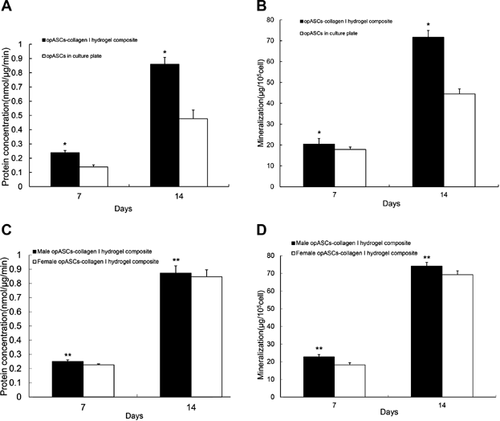
On days 7–14, ALPase activity in the opASCs-collagen I hydrogel composite was significantly higher than in the control opASCs cultures (P < 0.05, Figure 2a). Similar findings were made with the extent of extracellular mineralisation (Figure 2b). Higher levels of ALPase activity and extracellular mineralisation were seen in opASCs from male subjects than from female subjects (P < 0.05, Figures 2c and 2d).
Osteogenic specific genes expression
On day 1, expression of COL, OC and BSP was comparable between opASCs cultured in collagen I hydrogel and control cultures (P > 0.05, Figures 3a–3c). The mRNA level of COL increased progressively between days 4 and 14 in the opASCs-collagen I hydrogel composite, and was significantly higher than in the controls (P < 0.05).
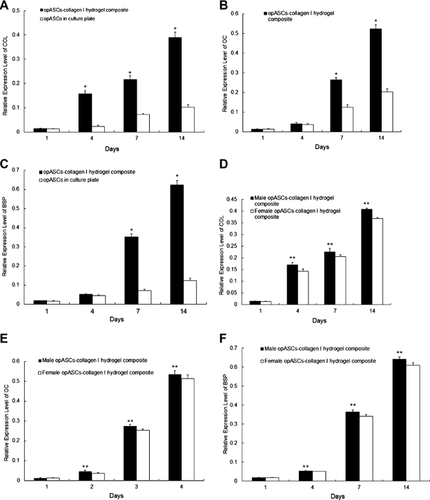
OC and BSP expression of opASCs in collagen I hydrogel was similar to that in controls on day 4, but became statistically higher on days 7 and 14 (P < 0.05, Figures 3b and c).
From day 4 onwards, COL, OC and BSP gene expression was consistently higher in opASCs from male subjects than from female subjects (P < 0.05, Figures 3d–3f).
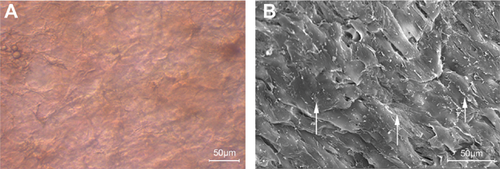
Morphological observations of opASCs-collagen I hydrogel composite
After 14 days of osteogenic culture, opASCs were evenly distributed in collagen I hydrogel and had a fibroblast-like appearance. The collagen I hydrogel acted as an intermediate medium (Figure 4a). The cells were closely packed at very high density, as seen by scanning electron microscopy (Figure 4b). Collagen I fibres were also present.
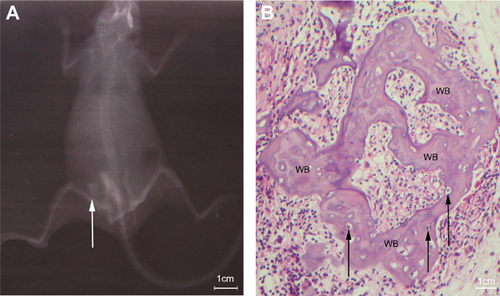
Ectopic bone formation
Four weeks after in vivo transplantation of both male and female composites, a radiopaque area appeared on the backs of the experimental nude mice, which provided evidence of new bone formation derived from the opASCs-collagen I hydrogel composite (Figure 5a). HE staining of the recovered composite showed the presence of woven bone with a trabecular structure together with osteocytes containing lacunae (Figure 5b). There were no obvious differences in the stages of osteogenesis obtained with male and female samples.
In vivo implantation of pure collagen I hydrogel resulted in complete absorption after 4 weeks (data not shown).
Discussion
BMSCs play a key role in bone maintenance; they act as progenitors for osteoblasts and regulators of osteoclastogenesis, as well as constitutively secrete a distinct set of cytokines with specialised functions in the microenvironment of the bone marrow (Caplan, 2005). The inherent characteristics of self-renewal and differentiation into osteogenic lineage make BMSCs a useful cell source for bone tissue engineering. However, the bone loss that accompanies the ageing process impairs the potential of BMSCs and results in decreased bone formation. Age-related decrease in the number, proliferative capacity and osteogenic potential of BMSCs occurs (Stolzing et al., 2008; Zhou et al., 2008). Specific genes involved with cell senescence, including p21 and p53, have been linked with age-related changes in BMSCs (Stolzing et al., 2008; Zhou et al., 2008). These physiological changes compromise the use of BMSCs in therapeutic bone tissue engineering in the elderly. However, ASCs may provide an ideal alternative source of postnatal progenitor cells with several advantages for the elderly, as their general potential remains almost intact as age increases. ASCs are superior to BMSCs, and have a sustained proliferation rate and adequate osteogenicity in elderly subjects (Chen et al., 2012; Wu et al., 2013). The activity of senescence-associated factors, such as p21 gene expression and beta galactosidase, are also maintained at a relatively lower level in ASCs than in BMCS (Chen et al., 2012). These findings suggest that ASCs should have priority over BMSCs in bone regenerative medicine, especially in view of an increasing elderly population.
Failure of internal fixation, such as screw loss or loosening, aseptic prosthetic loosening after arthroplasty, periprosthetic fractures and difficulty in treating bone defects resulting from heavy trauma or tumour resection, have all been closely connected with inherent bone failure in osteoporotic patients (Lee et al., 2012; Wu et al., 2012). Improvements in prosthesis design, augmentation of bone mass to provide resistance to screw loss, osteointegration around the periprosthetic region and reconstruction of bone defects, remain therapeutic priorities.
Injectable hydrogels facilitate orientation in minimally invasive orthopaedic procedures (Amini and Nair, 2012; Martinez-Sanz et al., 2012). Hydrogels are semisolid hydrophilic polymer networks that absorb >1000 times their dry weight in water. This property allows normal cell metabolism to take place with permeation of nutrients and oxygen and efflux of metabolites (Lutolf and Hubbell, 2005; Alexander and Shakesheff, 2006).
Hydrogels are completely biocompatible and biodegradable, and can be used to provide scaffolds capable of encapsulating large numbers of cells in bone regenerative medicine. These implants can be percutaneously injected to reinforce fixation and internal implant stability or to correct bone defects. The procedure is quick and simple, and results in only minimal damage to adjacent tissues. It also provides sufficient infusion of cell–hydrogel complex into the interspaces of sparse bone trabeculae, medullary cavities or cavity bone defects in osteoporotic patients.
We have used collagen I hydrogel as a cell carrier for opASCs, since type I collagen is a major organic component of bone matrices and acts as a regulator of cell adhesion and osteogenic differentiation. It enhances proliferation and osteogenic differentiation of MSCs (Hao et al., 2008). In accordance with previous studies, higher proliferation rate and increased osteogenesis after combining opASCs with collagen I hydrogel in vitro occurred. The transplantation of opASCs-collagen I hydrogel composite also resulted in new bone tissue formation in vivo. In vitro and in vivo experimental findings demonstrated that opASCs has good osteogenicity in culture and formed new bone tissue after being incorporated with collagen I hydrogel in an ectopic site.
Post-menopausal bone loss is closely related to the cessation of ovarian function, resulting in estrogen deficiency. However, in senile osteoporosis, which affects men as well as women, the underlying mechanism is probably connected with secondary hyperparathyroidism, gonadal sex steroid deficiency including testosterone and estrogen deficiency, bone marrow fat reduction and decreased bone formation (Demontiero et al., 2012). Although there are some different as well as overlapping aspects with reference to the etiology of osteoporosis between males and females, the reduced multipotent progenitor capacity of BMSCs that occurs during ageing ultimately alters the balance between osteoblasts and osteoclasts in favour of bone resorption and makes bone loss become a reality.
Gender differences may affect the overall capacity of MSCs. For example, bone marrow osteoblastic progenitors decrease more significantly with age in women than men (Muschler et al., 2001). In female subjects, BMSCs have greater differentiation potency towards adipogenesis and osteoclastogenesis than osteogenesis (Jiang et al., 2008). These differences were not seen in male subjects in the same study. Lower MSCs frequencies have also been observed in female mice donors than in males (Katsara et al., 2011).
The decreased cell number and multipotency of BMSCs has specifically limited its use in female subjects. Male ASCs differentiate more effectively than female ASCs towards the osteogenic lineage (Aksu et al., 2008). However, ASCs from female subjects were associated with consistently higher cell proliferation and surface marker expression than ASCs from males (Fossett et al., 2012). Female ASCs may have better proliferation ability than male ASCs (Shu et al., 2012).
Previous in vitro studies indicate that the doubling rate of female and male ASCs under osteogenic induction is similar (Chen et al., 2012). We found comparable growth curves in opASCs from female and male subjects induced in vitro with collagen I hydrogel. Although a relatively lower osteogenicity of female ASCs was found in vitro, no radiological or histological differences concerning ectopic bone formation were detected. This indicates that the gender differences in osteogenic potency of opASCs have little influence on bone formation in vivo after induction with hydrogel encapsulated in collagen I, and therefore show promise in the clinical perspective of opASCs, especially where female patients are concerned.
opASCs-collagen I hydrogel composites possess good osteogenic potency in vitro and in vivo. We are now investigating the feasibility of repairing cavity bone defects by opASCs-collagen I hydrogel composite in an animal model of osteoporosis.
Acknowledgement and funding
The authors acknowledge the funding support from Shandong Provincial Natural Science Foundation, China (ZR2012CQ018).
Conflict of interest
The authors have no conflicts of interest.