Matching-adjusted indirect comparison of isatuximab plus carfilzomib and dexamethasone with daratumumab plus lenalidomide and dexamethasone in relapsed multiple myeloma
Abstract
Backgound
Lenalidomide-based regimens are commonly used for early relapse in patients with relapsed and/or refractory multiple myeloma (RRMM) receiving at least one prior line of therapy. In the absence of head-to-head comparison, matching-adjusted indirect comparison (MAIC) was conducted to demonstrate efficacy and safety of isatuximab+carfilzomib+dexamethasone (Isa-Kd) versus daratumumab + lenalidomide + dexamethasone (Dara-Rd) in RRMM.
Methods
Patient-level data from IKEMA trial (Isa-Kd, n = 179) were matched to aggregate data from POLLUX (Dara-Rd, n = 286). Hazard ratios (HR) and 95% confidence intervals (CI) for progression-free survival (PFS) and overall survival (OS) were generated by weighted Cox proportional hazard models. Odds ratios (OR), 95% CI, and p-value were calculated for ≥very good partial response (≥VGPR) and treatment-emergent adverse events (TEAEs).
Results
After matching, no significant differences were observed between Isa-Kd and Dara-Rd in baseline characteristics except for patients with >3 prior lines (0.0% vs. 4.9%). Isa-Kd showed significantly better PFS (HR [95% CI]: 0.46 [0.24–0.86]; p = 0.0155), statistically non-significant improvement favoring Isa-Kd in OS (0.47 [0.20–1.09]; 0.0798), and ≥VGPR (OR [95% CI]: 1.53 [0.89–2.64]; p = 0.1252) than Dara-Rd. Odds of occurrence were significantly lower for some all-grade and grade 3/4 TEAEs with Isa-Kd than Dara-Rd.
Conclusion
These results support Isa-Kd as an efficacious treatment for early relapse in non-lenalidomide refractory patients.
1 INTRODUCTION
Despite being a rare form of cancer, multiple myeloma (MM) is the second most common hematologic malignancy characterized by excessive production of malignant monoclonal plasma cells.1-3 In 2020, nearly 176,400 newly diagnosed cases and about 117,000 MM-related deaths were reported globally.4 The US surveillance, Epidemiology, and End Results data registry estimated 34,920 new cases with 12,410 deaths related to MM in 2021.5 The incidence rate of MM in the United Kingdom is projected to increase by 11% between 2014 and 2035 to 12 cases per 100,000 by 2035.6, 7
The treatment landscape of MM has evolved over the last 10 years with the incorporation of immunomodulatory imid drugs (IMiDs, such as lenalidomide pomalidomide) and proteasome inhibitors (such as bortezomib and carfilzomib), which has resulted in improved overall survival (OS) and a prognosis with nearly 55.6% of diagnosed patients surviving for ≥5 years.5, 8, 9 The approval of anti-CD38 monoclonal antibodies (mAB) has further improved clinical outcomes in patients with relapsed and/or refractory multiple myeloma (RRMM).10 The CD38 type II transmembrane glycoprotein is abundantly expressed on MM cells, thus providing a unique target for CD38 antibodies in patients with MM.11 Currently, isatuximab (anti-CD38 IgG1 mAB) and daratumumab (anti-CD38 human IgG-κ mAB) are the only two anti-CD38 mABs approved for the treatment of RRMM. Based on the results of the phase 3 IKEMA trial (NCT03275285), isatuximab in combination with carfilzomib and dexamethasone (Isa-Kd) was approved by the US FDA on March 31, 2021, for the treatment of adult patients with RRMM who have received one to three prior lines of therapy (LoT),12-14 and by the European Medicine Agency on April 19, 2021, for the treatment of adult patients with MM who have received at least one prior therapy.15 Daratumumab, in combination with lenalidomide and dexamethasone (Dara-Rd), was approved by the US FDA on November 21, 2016 and by European Medicine Agency on May 20, 2016, for the treatment of adult patients with RRMM16-18 and MM19 who have received at least one prior therapy, respectively. More recently, daratumumab, in combination with carfilzomib and dexamethasone (Dara-Kd), was approved by the US FDA on August 20, 2020 to treat adult patients with RRMM who have received one to three LoT.20
The choice of a treatment regimen for patients with RRMM depends upon several factors, such as response toward prior therapies, patient condition, expected effectiveness, and tolerability.21 The current literature highlights that lenalidomide-based regimens are frequently used in non-lenalidomide-refractory early relapse patients until progression in the second line (2L+) setting.22-24 A Flatiron Health Database study revealed that lenalidomide-based regimens are the most commonly used treatment option in patients with MM, as monotherapy or combination therapy (with PI or anti-CD38 mAB), despite experiencing relapse on lenalidomide.22 For optimum effect, doublet or triplet-based regimens are usually preferred over single agents for patients with RRMM.21 In the 2L+ setting, triple combination therapy with mAB + IMiD + dexamethasone, such as Dara-Rd is a go-to regimen for patients at first relapse, and it is frequently used in non-lenalidomide-refractory patients until progression, whereas Isa-Kd is usually considered for lenalidomide-refractory patients.23-25
Despite advances in therapy with improved survival outcomes, MM remains largely incurable, with patients continuously relapsing over the course of the disease.9, 26, 27 Over time, patients can become refractory or resistant to the existing therapies, that is, develop RRMM.9, 26, 27 A retrospective evaluation of data on patients with MM treated at Mayo Clinic between January 2006 and December 2014 revealed that 82 (16.0%) patients relapsed within 12 months (median: 8.0 (95% CI: 6.3–8.9)) of starting initial therapy with autologous stem cell transplant and induction regimens, such as IMiDs, proteasome inhibitors, or both.28 An International Myeloma Working Group study that enrolled 543 patients with MM who had received at least three prior LoT (median: 4 [3–13]) highlighted that the median survival for the entire cohort was relatively low, that is, 13 months.29 Likewise, a real-world retrospective study of patients with MM who were refractory to IMiDs and proteasome inhibitors or who had received three or more prior LoT, including a PI and an IMiD, reported a median OS of only 8 months approximately.30
Evidence of comparative efficacy is crucial to demonstrate treatment value and aid clinical decision-making in choosing the optimal treatment regimen. In the absence of published evidence from head-to-head clinical trials and without a common comparator between the clinical trials, matching-adjusted indirect comparison (MAIC) was used to compare the efficacy and safety of two approved anti-CD38 mABs, that is, Isa-Kd versus Dara-Rd, to inform treatment choice in patients with RRMM at their first relapse. The National Institute for Health and Care Excellence support document outlines the MAIC approach for comparing relative treatment effects between two interventions31 when direct treatment comparison is not feasible.32, 33 Recently, the MAIC methodology has provided valuable insights into the relative efficacy of different oncology treatment options, including RRMM.34, 35
The primary objective of this MAIC analysis was to compare the progression-free survival (PFS), OS, and very good partial response (VGPR) or better of Isa-Kd with that of Dara-Rd. The secondary objective was to assess the safety of Isa-Kd versus that of Dara-Rd in patients with RRMM.
2 METHODS
2.1 Data sources
In this MAIC analysis, individual patient-level data (IPD) for Isa-Kd were obtained from the IKEMA trial (median follow-up: 20.7 months).14, 36 In brief, IKEMA is a multinational, prospective, randomized, open-label, parallel-group, phase 3 study conducted at 69 study centers in 16 countries across North America, South America, Europe, and the Asia-Pacific region. Patients with RRMM, aged at least 18 years, who had received one to three previous LoT and had measurable evidence of disease (serum M-protein ≥0.5 g/dL or urine M-protein ≥200 mg/24 h) were eligible for enrollment in the IKEMA trial. The complete list of inclusion and exclusion criteria of the IKEMA trial has been published previously.14, 36 A total of 302 eligible patients were randomly assigned in a 3:2 ratio to receive either Isa-Kd (i.e., isatuximab group; n = 179) or carfilzomib and dexamethasone (Kd) (i.e., control group; n = 123). For patients assigned to the isatuximab group, isatuximab at a dose of 10 mg/kg was administered intravenously (i.v.) on days 1, 8, 15, and 22 in the first 28-day cycle and on days 1 and 15 in the following cycles. Patients in both treatment groups received carfilzomib (20 mg/m2, i.v.) on days 1 and 2 of cycle 1; 56 mg/m2 on days 8, 9, 15, and 16 of cycle 1; and then 56 mg/m2 on days 1, 2, 8, 9, 15, and 16 of the following cycles. Dexamethasone (20 mg, i.v. or orally) was administered on days 1, 2, 8, 9, 15, 16, 22, and 23 of every cycle.14, 36 The primary efficacy endpoint of IKEMA was PFS, and key secondary efficacy endpoints were overall response rate, rate of VGPR or better, and OS. The other secondary endpoints, including safety, were also assessed.14, 36
For Dara-Rd, the published aggregate data were obtained from the POLLUX trial publications.18, 37 In brief, POLLUX is a randomized, open-label, multicenter, active-controlled, parallel-group, phase 3 study conducted at 135 sites in 18 countries across North America, Europe, and the Asia-Pacific region. Patients with RRMM who had received one or more lines of previous therapy and had measurable disease at screening were eligible for enrollment in the POLLUX trial. The complete list of inclusion and exclusion criteria of the POLLUX trial has been published previously.18 A total of 569 eligible patients were randomly assigned in a 1:1 ratio to receive either Dara-Rd (i.e., daratumumab group; n = 286) or lenalidomide and dexamethasone (Rd) (i.e., control group; n = 283). For patients who were assigned to the daratumumab group, daratumumab at a dose of 16 mg/kg was administered i.v. on days 1, 8, 15, and 22 for 8 weeks during cycles 1 and 2; every 2 weeks (on days 1 and 15) for 16 weeks (cycles 3 through 6); and every 4 weeks subsequently. Patients in both treatment groups received lenalidomide (25 mg, orally) on days 1 to 21 of each cycle and dexamethasone (40 mg, orally) once every week.18 The primary efficacy endpoint of POLLUX was PFS, and secondary efficacy endpoints included time to progression, overall response rate, rate of VGPR or better, rate of complete response (CR) or better, and OS. Additionally, adverse events were also monitored.18
2.2 Feasibility assessment
A feasibility assessment was conducted to evaluate key points of similarity and heterogeneity (i.e., cross-trial imbalances) between the IKEMA and POLLUX trials, including trial design, treatment arms, patient population, inclusion and exclusion criteria, sample size, outcome definitions, and assessments. This assessment revealed that the study design, outcome definitions, and eligibility criteria were broadly similar between the IKEMA and POLLUX trials. Furthermore, there was sufficient overlap between the summarized baseline characteristics reported in the primary POLLUX trial publication18 and the available measures in IKEMA IPD.37, 38 Therefore, an unanchored MAIC between Isa-Kd and Dara-Rd was deemed feasible based on the IKEMA and corresponding POLLUX trial/arm data for PFS, OS, VGPR or better, and treatment-emergent adverse events (TEAEs). However, some key differences were identified in the eligibility criteria between these two trials. Therefore, to emulate the POLLUX trial population, additional eligibility criteria of POLLUX (creatinine clearance ≥30 mL/min, hemoglobin >7.5 g/dL, platelet count ≥75 × 109/L, and non-lenalidomide-refractory) were applied to the IKEMA Isa-Kd IPD.
2.3 Comparison of baseline characteristics and outcomes before matching
The baseline characteristics were summarized and compared between the Isa-Kd population of IKEMA and the Dara-Rd population of POLLUX, using Wald and chi-square tests (or Fisher's exact test for small frequencies) for continuous and categorical variables, respectively.
The outcomes of interest assessed in the MAIC analysis were based on the primary and secondary endpoints of the IKEMA and POLLUX trials. The endpoints included PFS, OS, VGPR or better, as well as all-grade and grade 3/4 TEAEs. For PFS39 and OS,18 IPD for Isa-Kd was used from the IKEMA trial, and data from the published Kaplan-Meier (KM) curves for Dara-Rd were extracted via digitization software.40 Based on the extracted survival curves, reported numbers of events, and patients at risk at various time points in the publications, a pseudo-IPD was reconstructed using the approach developed by Guyot et al.41 Before matching, PFS and OS were summarized using the KM curves in addition to the median PFS and OS (in months), and log-rank tests were conducted to compare the Isa-Kd and Dara-Rd arms. The HR and corresponding 95% CI and p-values were estimated from Cox proportional hazards models.
The VGPR or better (calculated as the proportion of patients with CR, stringent CR, or VGPR as the best overall response) was obtained from the IPD for Isa-Kd and from the POLLUX publication with the closest follow-up to the IKEMA trial that reported this outcome.14, 37 ORs were reported, and the corresponding 95% CI and p-value were calculated using the Wald tests to compare between comparators before matching.
The all-grade TEAEs and all-grade 3 or 4 TEAEs with rates >5% reported for the Dara-Rd arm of the POLLUX trial37 were included. The data collection and analysis of four hematological adverse events (i.e., neutropenia, anemia, thrombocytopenia, and lymphopenia) were different in POLLUX and IKEMA. In POLLUX laboratory, data were reported as TEAEs while in IKEMA all laboratory tests (normal and abnormal) were to be reported and the worst grade was calculated from all collected laboratory values. Therefore, they were excluded from the MAIC analyses. The OR, 95% CI, and p-values were calculated before matching.
2.4 Matching average baseline characteristics
The aggregate baseline characteristics (average or proportions) were matched between the Isa-Kd and Dara-Rd patients. Specifically, individual patients in the IKEMA trial were assigned weights such that the weighted mean (or proportion) baseline characteristics for patients in the Isa-Kd arm exactly matched those reported for patients in the Dara-Rd arm, and each patient's weight was equal to their estimated odds (propensity) of being in the POLLUX trial versus the IKEMA trial. The weights meeting these conditions were obtained from a logistic regression model for the propensity of enrollment in the POLLUX trial versus the IKEMA trial. All matched-on baseline characteristics were included as predictors in the model.
The matched-on baseline characteristics were prioritized for inclusion in the Isa-Kd versus Dara-Rd MAIC by internal medical and statistics experts based on clinical importance (i.e., the potential for treatment modification) and major trial differences. The matched-on characteristics included age (≤64, 65–74, and ≥75 years), the Eastern Cooperative Oncology Group performance status (0; 1–2), number of prior therapy lines (1 or ≥2), disease stage (I, II, or III per the International Staging System) at entry, cytogenetic risk (standard, high, or unknown), prior treatment (PI, lenalidomide, and IMiD), and refractory status (PI-refractory only or IMiD-refractory only). The other baseline characteristics reported/included but not matched were gender (%male), race/ethnicity, geographic region, creatinine clearance levels, previous treatments (autologous stem cell transplant, or alkylating agent), refractoriness to PI, IMiD, refractoriness to the previous line, and years from diagnosis to randomization.
2.5 Comparisons of baseline characteristics and outcomes after matching
Post-matching adjustments, the baseline characteristics were compared between the Isa-Kd and Dara-Rd arms; p-values for continuous and categorical variables were calculated using the Wald tests. Effective sample size (ESS) was also calculated.
For PFS and OS, the KM curves were estimated for Isa-Kd after matching by incorporating the matching weights and compared with Dara-Rd. The median PFS or OS was calculated from the estimated KM curves. Weighted log-rank tests were conducted to compare Isa-Kd with Dara-Rd after matching. The HRs, including 95% CI and p-values for PFS and OS, were estimated using a weighted Cox proportional hazards model based on the weighted sample of Isa-Kd and the pseudo-IPD of the comparator. The proportional hazards assumption tests were performed based on the scaled Schoenfeld residuals, and the corresponding p-values were reported. The OR and corresponding 95% CI and p-values for VGPR or better were calculated using the Wald tests. Likewise, the OR, 95% CI, and p-values were calculated for all-grade and grade 3/4 TEAEs.
3 RESULTS
3.1 Patient demographic and baseline characteristics
The original sample size of the Isa-Kd arm of the IKEMA trial was 179, of which 112 patients were included in this MAIC analysis and were considered for weighting following the imposition of additional eligibility criteria of POLLUX. A total of 286 patients were included in the Dara-Rd arm of the POLLUX trial (Table 1).
| Characteristics | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Isa-Kd | Dara-Rd | p-value* | Isa-Kd | Dara-Rd | p-value** | |
| N = 112 | N = 286 | ESS = 66.2 | N = 286 | |||
| Age (years) | ||||||
| Mean ± SD | 64.2 ± 9.1 | 64.4 ± 9.0 | 0.8550 | 64.3 ± 9.1 | 64.4 ± 9.0 | 0.8968 |
| <65b, n (%) | 50 (44.6%) | 133 (46.5%) | 0.8235 | 30.8 (46.5%) | 133 (46.5%) | ~ |
| 65–74b, n (%) | 51 (45.5%) | 124 (43.4%) | 0.7783 | 28.7 (43.4%) | 124 (43.4%) | ~ |
| ≥75b, n (%) | 11 (9.8%) | 29 (10.1%) | 1.0000 | 6.7 (10.1%) | 29 (10.1%) | ~ |
| Gender | ||||||
| Male, n (%) | 59 (52.7%) | 173 (60.5%) | 0.1908 | 37.7 (57.0%) | 173 (60.5%) | 0.6083 |
| Race/ethnicity, n (%) | ||||||
| White | 82 (73.2%) | 207 (72.4%) | 0.9654 | 50.7 (76.6%) | 207 (72.4%) | 0.4640 |
| Asian | 18 (16.1%) | 54 (18.9%) | 0.61 | 9.7 (14.6%) | 54 (18.9%) | 0.3761 |
| Black | 3 (2.7%) | 5 (1.7%) | 0.6919 | 2.9 (4.4%) | 5 (1.7%) | 0.3689 |
| Other | 9 (8.0%) | 20 (7.0%) | 0.8843 | 2.9 (4.4%) | 20 (7.0%) | 0.2715 |
| Geographic region, n (%) | ||||||
| Europe | 56 (50.0%) | 150 (52.5%) | 0.743 | 35.7 (53.9%) | 150 (52.5%) | 0.8283 |
| North America | 14 (12.5%) | 38 (13.3%) | 0.9649 | 10.9 (16.5%) | 38 (13.3%) | 0.5568 |
| Rest of the world | 42 (37.5%) | 98 (34.3%) | 0.6235 | 19.5 (29.5%) | 98 (34.3%) | 0.4363 |
| ECOG performance status, n (%) | ||||||
| 0b | 61 (54.5%) | 139 (48.6%) | 0.3470 | 32.2 (48.6%) | 139 (48.6%) | ~ |
| 1 or 2b | 50 (44.6%) | 147 (51.4%) | 0.2710 | 34.0 (51.4%) | 147 (51.4%) | ~ |
| Number of previous lines of therapy, n (%) | ||||||
| 1b | 66 (58.9%) | 149 (52.1%) | 0.2637 | 34.5 (52.1%) | 149 (52.1%) | ~ |
| 2 | 34 (30.4%) | 85 (29.7%) | 0.9976 | 20.3 (30.7%) | 85 (29.7%) | 0.8804 |
| 3 | 12 (10.7%) | 38 (13.3%) | 0.5974 | 11.4 (17.2%) | 38 (13.3%) | 0.4899 |
| >3 | 0 (0.0%) | 14 (4.9%) | 0.0134**** | 0.0 (0.0%) | 14 (4.9%) | 0.0134**** |
| 2 or 3 | 46 (41.1%) | 123 (43.0%) | 0.8115 | 31.7 (47.9%) | 123 (43.0%) | 0.4759 |
| ≥2b | 46 (41.1%) | 137 (47.9%) | 0.2637 | 31.7 (47.9%) | 137 (47.9%) | ~ |
| ≥3 | 12 (10.7%) | 52 (18.2%) | 0.0945 | 11.4 (17.2%) | 52 (18.2%) | 0.8685 |
| Disease stage at study entry, International staging system (ISS), n (%) | ||||||
| I | 61 (54.5%) | 137 (47.9%) | 0.2864 | 32.0 (48.3%) | 137 (47.9%) | 0.9538 |
| II | 37 (33.0%) | 93 (32.5%) | 1.0000 | 21.3 (32.1%) | 93 (32.5%) | 0.9503 |
| IIIb | 14 (12.5%) | 56 (19.6%) | 0.1280 | 13.0 (19.6%) | 56 (19.6%) | ~ |
| I or IIb | 98 (87.5%) | 230 (80.4%) | 0.1280 | 53.2 (80.4%) | 230 (80.4%) | ~ |
| Cytogenetic risk, n (%) | ||||||
| Highb,*** | 27 (24.1%) | 35 (12.2%) | 0.0054**** | 8.1 (12.2%) | 35 (12.2%) | ~ |
| Standardb | 72 (64.3%) | 193 (67.5%) | 0.6242 | 44.7 (67.5%) | 193 (67.5%) | ~ |
| Unknownb | 13 (11.6%) | 58 (20.3%) | 0.0592 | 13.4 (20.3%) | 58 (20.3%) | ~ |
| Creatinine levels, n (%) | ||||||
| ≤60 mL/min | 29 (25.9%) | 80 (28.0%) | 0.7693 | 18.3 (27.7%) | 80 (28.0%) | 0.9683 |
| >60 mL/min | 83 (74.1%) | 199 (69.6%) | 0.4407 | 47.9 (72.3%) | 199 (69.6%) | 0.6715 |
| Missing | 0 (0.0%) | 7 (2.4%) | 0.1982 | 0.0 (0.0%) | 7 (2.4%) | 0.1982 |
| Previous treatments, n (%) | ||||||
| ASCT | 69 (61.6%) | 180 (62.9%) | 0.8955 | 36.4 (54.9%) | 180 (62.9%) | 0.2351 |
| Alkylating agents | 105 (93.8%) | 268 (93.7%) | 1.0000 | 61.2 (92.4%) | 268 (93.7%) | 0.7307 |
| PIb | 104 (92.9%) | 245 (85.7%) | 0.0728 | 56.8 (85.7%) | 245 (85.7%) | ~ |
| IMiDb | 70 (62.5%) | 158 (55.2%) | 0.2289 | 36.6 (55.2%) | 158 (55.2%) | ~ |
| Lenalidomideb | 12 (10.7%) | 50 (17.5%) | 0.1283 | 11.6 (17.5%) | 50 (17.5%) | ~ |
| Refractory disease, n (%) | ||||||
| Refractory to PI onlyb | 19 (17.0%) | 57 (19.9%) | 0.5926 | 13.2 (19.9%) | 57 (19.9%) | ~ |
| Refractory to IMiD onlyb | 11 (9.8%) | 10 (3.5%) | 0.0221**** | 2.3 (3.5%) | 10 (3.5%) | ~ |
| Refractory to PI and IMiD | 7 (6.3%) | 7 (2.4%) | 0.1213 | 4.2 (6.3%) | 7 (2.4%) | 0.1617 |
| Refractory to previous line | 39 (34.8%) | 80 (28.0%) | 0.2223 | 25.8 (39.0%) | 80 (28.0%) | 0.0968 |
| Time since diagnosis | ||||||
| Years from diagnosis to randomization (≥Dara-Rd median of 3.5) | 50 (44.6%) | 143 (50.0%) | 0.3953 | 33.5 (50.6%) | 143 (50.0%) | 0.9356 |
- Abbreviations: ASCT, autologous stem cell transplant; Dara-Rd, daratumumab + lenalidomide + dexamethasone; ECOG, Eastern Cooperative Oncology Group; IMiD, immunomodulatory drug; ESS, effective sample size; Isa-Kd, isatuximab + carfilzomib + dexamethasone; MAIC, matching-adjusted indirect comparison; min, minute; PI, proteasome inhibitor; SD, standard deviation.
- a Means and standard deviations are shown for continuous characteristics; percentages are shown for categorical characteristics.
- b Characteristics matched on in MAIC.
- * Before matching, p-values for continuous variables are calculated using the Wald test. p-Values for categorical variables are calculated using the chi-square test. The Fisher's exact test is used for categorical variables with small frequency (i.e., n < 5)
- ** After matching: All p-values are calculated using the Wald test. The p-value is not calculated for matched variables; instead, a “~” is shown
- *** High-risk cytogenetic group in both POLLUX and IKEMA includes patients with del(17p) and/or translocation t(4;14) and/or translocation t(14;16)
- **** p-Value < 0.05.
Before matching but after applying the POLLUX inclusion criteria, statistically significant differences (p < 0.05) were observed between the Isa-Kd and Dara-Rd populations in the percentage of patients with more than three prior LoT (0.0% vs. 4.9%), high cytogenetic risk (24.1% vs. 12.2%), and who were refractory to IMiD only (9.8% vs. 3.5%). After MAIC weighting, all baseline characteristics were well balanced between Isa-Kd and Dara-Rd populations, except for patients with more than three prior LoT (0.0% vs. 4.9%). Additionally, a balance was also maintained in the factors, which were not matched-on as indicated by the p-values.
Based on the weighting of the observations, the ESS adjusts the sample size to show the amount of overlap in patient baseline characteristics between the study populations included in the MAIC analysis.42 After matching, the sample size was reduced by 40.8%. The ESS of Isa-Kd was 66.2, reflecting the approximate number of patients with an overlap of baseline characteristics with theDara-Rd arm of the POLLUX trial (n = 286).
3.2 Progression-free survival
The proportional hazards assumption was tested before (χ2 = 0.01, p = 0.92) and after (χ2 = 1.47, p = 0.23) matching, and the results showed that the assumption was not violated (Figure 1).
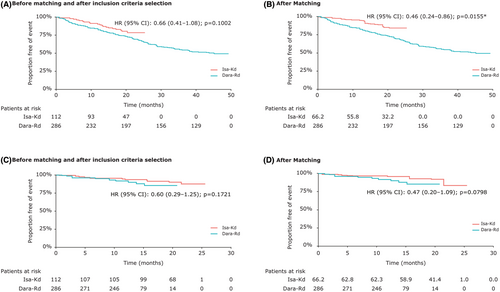
The pre- and post-matched PFS HRs of Isa-Kd versus Dara-Rd were 0.66 (95% CI: 0.41–1.08; p = 0.1002, Figure 1A) and 0.46 (95% CI: 0.24–0.86; p = 0.0155, Figure 1B). A significant difference was observed in the PFS HR between the two treatments after matching in favor of Isa-Kd.
3.3 Overall survival
The proportional hazards assumption was tested before (χ2 = 0.11, p = 0.74) and after (χ2 = 0.19, p = 0.66) matching, and the results showed that the assumption was not violated (Figure 1).
The pre- and post-matched OS HRs of Isa-Kd versus Dara-Rd were 0.60 (95% CI: 0.29–1.25; p = 0.1721, Figure 1C) and 0.47 (95% CI: 0.20–1.09; p = 0.0798, Figure 1D). No significant differences were observed in the OS HR between the two treatments before and after matching. However, a numerical trend was observed for OS in favor of Isa-Kd compared with Dara-Rd after matching adjustment.
3.4 Very good partial response or better
Before matching adjustment, a similar proportion of patients receiving Isa-Kd and Dara-Rd achieved VGPR or better (78.6% vs. 78.7%; OR = 1.00; p = 0.9837). Post-MAIC weighting, a statistically non-significant improvement in the rate of VGPR or better was observed in favor of Isa-Kd (84.9% vs. 78.7%; OR = 1.53, p = 0.1252) compared with Dara-Rd (Table 2).
| Arm | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| VGPR or better | 95% CI | VGPR or better | 95% CI | |||
| Dara-Rd | 78.7% | (73.4%–83.3%) | 78.7% | (73.4%–83.3%) | ||
| Isa-Kd | 78.6% | (70.4%–85.5%) | 84.9% | (76.1%–90.9%) | ||
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p-value | Odds ratio | 95% CI | p-value | |
| Isa-Kd versus Dara-Rd | 1.00 | (0.64–1.54) | 0.9837 | 1.53 | (0.89–2.64) | 0.1252 |
- Abbreviations: CI, confidence interval; Dara-Rd, daratumumab + lenalidomide + dexamethasone; Isa-Kd, isatuximab + carfilzomib + dexamethasone; VGPR, very good partial response.
3.5 Safety outcomes
After MAIC weighting, TEAEs leading to treatment discontinuations were higher among patients receiving Dara-Rd than those receiving Isa-Kd (12.0% vs. 9.0%), although the difference was not significant (p = 0.4712) (Table 3). Treatment with Isa-Kd, after matching, was associated with significantly (p < 0.05) lower rates of all-grade constipation, cough, diarrhea, fatigue, febrile neutropenia, muscle spasms, nasopharyngitis, nausea, and pyrexia than Dara-Rd (Table 3). Conversely, a higher rate of asthenia, bronchitis, dyspnea, headache, pneumonia, and upper respiratory tract infections was observed in the Isa-Kd arm than in the Dara-Rd arm, although the difference was not significant. Likewise, the proportion of patients reporting back pain, edema peripheral, insomnia, and vomiting was higher in the Dara-Rd arm than in the Isa-Kd arm; observed differences were not significant (Table 3). Post-matching, rates of grade 3 or 4 diarrhea (0.6% vs. 7.1%; p = 0.0002) and febrile neutropenia (0.3% vs. 6.0%; p = 0.0045) were significantly lower in the Isa-Kd than in the Dara-Rd arm. Conversely, a higher rate of grade 3 or 4 pneumonia was observed in the Isa-Kd arm than in the Dara-Rd arm, whereas the proportion of grade 3 or 4 fatigue was higher in the Dara-Rd arm than in the Isa-Kd arm, though these differences were not significant (Table 3).
| Isa-Kd | Dara-Rd | Odds ratio | p-value | |
|---|---|---|---|---|
| TEAE rate (95% CI) | TEAE rate (95% CI) | (95% CI) | ||
| Any TEAE leading to treatment discontinuation | 9.0% (3.9%–19.4%) | 12.0% (8.5%–16.4%) | 0.73 (0.30–1.73) | 0.4712 |
| All-grade TEAEs | ||||
| Asthenia | 21.1% (12.5%–33.5%) | 18.0% (13.7%–23.0%) | 1.22 (0.69–2.17) | 0.4999 |
| Back pain | 20.2% (11.9%–32.3%) | 20.5% (15.9%–25.7%) | 0.98 (0.56–1.74) | 0.9561 |
| Bronchitis | 19.1% (11.5%–29.8%) | 18.7% (14.4%–23.8%) | 1.02 (0.59–1.77) | 0.9378 |
| Constipation | 2.1% (0.8%–5.4%) | 31.1% (25.7%–36.8%) | 0.05 (0.02–0.13) | <0.0001* |
| Cough | 17.0% (9.0%–29.9%) | 32.2% (26.7%–37.9%) | 0.43 (0.23–0.83) | 0.0110* |
| Diarrhea | 32.6% (22.4%–44.7%) | 50.9% (44.9%–56.8%) | 0.47 (0.31–0.71) | 0.0004* |
| Dyspnea | 27.7% (17.8%–40.3%) | 20.9% (16.3%–26.1%) | 1.45 (0.88–2.39) | 0.1424 |
| Edema peripheral | 13.5% (7.0%–24.4%) | 18.7% (14.4%–23.8%) | 0.68 (0.34–1.34) | 0.2631 |
| Fatigue | 23.7% (15.5%–34.5%) | 36.4% (30.8%–42.3%) | 0.54 (0.34–0.86) | 0.0087* |
| Febrile neutropenia | 0.3% (0.0%–2.4%) | 6.0% (3.5%–9.4%) | 0.05 (0.01–0.40) | 0.0045* |
| Headache | 17.9% (10.0%–30.0%) | 15.2% (11.2%–19.9%) | 1.22 (0.65–2.30) | 0.5387 |
| Insomnia | 17.1% (10.1%–27.5%) | 23.7% (18.8%–29.1%) | 0.66 (0.38–1.16) | 0.1522 |
| Muscle spasms | 13.4% (6.4%–25.8%) | 28.6% (23.4%–34.3%) | 0.38 (0.18–0.81) | 0.0113* |
| Nasopharyngitis | 14.0% (7.7%–24.2%) | 29.7% (24.4%–35.4%) | 0.39 (0.21–0.72) | 0.0025* |
| Nausea | 12.7% (6.9%–22.3%) | 26.9% (21.8%–32.4%) | 0.40 (0.21–0.75) | 0.0043* |
| Pneumonia | 22.3% (14.3%–33.0%) | 20.5% (15.9%–25.7%) | 1.11 (0.68–1.83) | 0.6725 |
| Pyrexia | 7.3% (3.0%–17.0%) | 23.7% (18.8%–29.1%) | 0.26 (0.10–0.64) | 0.0034* |
| Upper respiratory tract infection | 41.8% (30.5%–54.1%) | 37.1% (31.5%–43.0%) | 1.22 (0.82–1.81) | 0.3306 |
| Vomiting | 11.9% (5.9%–22.6%) | 18.4% (14.0%–23.4%) | 0.60 (0.29–1.25) | 0.1726 |
| Grade 3 or 4 TEAEs | ||||
| Diarrhea | 0.6% (0.2%–2.2%) | 7.1% (4.4%–10.7%) | 0.08 (0.02–0.31) | 0.0002* |
| Fatigue | 3.0% (0.9%–9.4%) | 6.4% (3.8%–9.9%) | 0.46 (0.13–1.59) | 0.2199 |
| Febrile neutropenia | 0.3% (0.0%–2.4%) | 6.0% (3.5%–9.4%) | 0.05 (0.01–0.40) | 0.0045* |
| Pneumonia | 16.1% (9.2%–26.6%) | 12.0% (8.5%–16.4%) | 1.40 (0.75–2.64) | 0.2921 |
- Abbreviations: CI, confidence interval; Dara-Rd, daratumumab + lenalidomide + dexamethasone; Isa-Kd, isatuximab + carfilzomib + dexamethasone; TEAE, treatment-emergent adverse event.
- * Statistically significant at a 0.05 level.
4 DISCUSSION
In the current clinical practice, lenalidomide-based regimens, such as Dara-Rd, are considered the go-to regimen in the 2 L+ setting for patients with RRMM who experienced the first relapse and are frequently used in non-lenalidomide-refractory patients until progression.22-24 Immunotherapy drugs are being broadly used in the treatment of RRMM. Daratumumab was the first anti-CD38 mAb to receive the FDA approval for patients with MM,43 later joined by another anti-CD38 mAb, isatuximab, approved for use in patients with RRMM.12 Given the absence of published evidence from head-to-head clinical trials, we conducted the current MAIC analysis in patients with RRMM to understand the comparative efficacy and safety of Isa-Kd versus Dara-Rd. This MAIC analysis suggests that Isa-Kd may represent an important new treatment option offering improved efficacy outcomes with a tolerable safety profile for non-lenalidomide refractory patients at early relapse compared with Dara-Rd, a lenalidomide-based regimen.
Matching-adjusted indirect comparison can adjust for differences between trial populations that are potential baseline treatment effect modifiers and prognostic factors that might otherwise bias the comparison.32, 44 After matching adjustment, all baseline characteristics were well balanced between the Isa-Kd and Dara-Rd populations except for patients with more than three prior LoT (0.0% vs. 4.9%) as it was not possible to match this factor given that the IKEMA trial did not include patients with more than three prior LoT. However, this difference is not likely to significantly impact the MAIC results, given the small number of these patients.
In this MAIC analysis, PFS was significantly better with Isa-Kd than Dara-Rd (pre- and post-match HR: 0.66; p = 0.1002 vs. 0.46; p = 0.0155) after adjusting for differences in the inclusion criteria and baseline characteristics. The matching adjustment increased the estimated benefit of Isa-Kd versus Dara-Rd for OS (pre- and post-match HR: 0.60; p = 0.1721 vs. 0.47; p = 0.0798), although it was statistically non-significant. The existing literature suggests that an improved response (e.g., VGPR rate) is an important indicator of prognosis in patients with MM and is correlated with improvements in other efficacy outcomes, such as PFS and OS.45 A numerical improvement in VGPR or better rates was observed with Isa-Kd compared with Dara-Rd, although it was statistically non-significant (pre- and post-match OR: 1.00; p = 0.9837 vs. 1.53; p = 0.1252). The comparative efficacy results from this MAIC analysis should be interpreted with the caveat as the results from both clinical trials are immature. It will be important to update the recent analyses as extended follow-up data become available to understand the long-term survival estimates better.
In terms of safety profile, Isa-Kd was well tolerated and demonstrated significantly lower rates of several all-grade (0.3% vs. 6.0%; p = 0.0045) and grade 3 or 4 (0.3% vs. 6.0%; p = 0.0045) TEAEs than Dara-Rd. Although higher rates were observed for a few TEAEs with Isa-Kd than Dara-Rd, the differences were statistically non-significant. Febrile neutropenia is a recognized complication in cancer treatment that could result in hospitalization and could substantially impact treatment outcomes due to delays in a treatment cycle, dose reductions, or treatment discontinuations.46 A retrospective chart review study among adult patients with cancer and diagnosis of febrile neutropenia between 2010 and 2013 reported a hospital mortality rate of 17.3% and the 30-day mortality rate of 20.5%, suggesting that febrile neutropenia in hospitalized patients could result in a significant mortality rate.47 In combination with dexamethasone, lenalidomide is one of the most frequently used treatment options in patients with MM though this combination has reported one of the highest incidences of neutropenia.48 Additionally, neutropenia has been predominantly reported for Dara-Rd.48 In the POLLUX trial, a higher proportion of patients in the daratumumab group (51.9%) reported neutropenia than those in the control group, that is, Rd (37.0%).18 Though the rationale for this mechanism remains unclear, the target of daratumumab, CD38 (target epitope is different than that of isatuximab), is known to regulate neutrophil chemotaxis and is present on myeloid stem cells.48-50
Both isatuximab and daratumumab are anti-CD38 mAb; however, isatuximab differentiates itself from daratumumab in terms of key mechanistic differences. At present, isatuximab is the only anti-CD38 mAb that could induce direct apoptosis in MM cells, although only in patients with elevated CD38 expression, whereas daratumumab may induce apoptosis, but only in the presence of cross-linking agents.13, 51-55 Isatuximab was shown to inhibit CD38 enzymatic activity more effectively than daratumumab.56, 57 In terms of the dosing schedule, isatuximab is administered every other week starting from cycle 2, whereas daratumumab is started from cycle 3.14, 18, 36 After six cycles, isatuximab is continued to be administered every other week, whereas daratumumab is administered once a month.12, 17 Additionally, the difference between carfilzomib and lenalidomide might have contributed to better efficacy outcomes with Isa-Kd than with Dara-Rd.58 Some of these possibilities, including the mechanism of action, dosing schedule of isatuximab, and enhanced antitumor effects of combination therapies, might have contributed to the augmented efficacy of Isa-Kd compared with Dara-Rd. However, further comparative evidence is warranted to draw definite conclusions.
The association of mAbs targeting CD38 with carfilzomib has been well studied. Two randomized phase 3 trials, that is, IKEMA (Kd vs. Kd with isatuximab) and CANDOR (Kd vs. Kd with daratumumab), are quite similar with a CD38-directed mAb paired with the Kd backbone (control arm) in patients with RRMM with a median of two prior LoT.14, 59 Both trials have a similar design, and the proportion of lenalidomide-refractory patients (32%–33%) was also comparable.14, 59 Although cross-trial comparisons of other anti-CD38 PI triplet combinations in this patient population should be interpreted with caution, the HR for PFS in the isatuximab group of IKEMA (median follow-up 20.7 months: HR [95% CI]: 0.53 [0.32–0.89]) was numerically more favorable than in the CANDOR study (median follow-up 16.9 months, HR [95% CI]: 0.63 [0.46–0.85]; median follow-up 28.6 months, HR [95% CI]: 0.59 [0.45–0.78]).14, 59, 60 However, definite conclusions can only be drawn when longer follow-up data will be available for both the IKEMA and CANDOR trials.
There are several limitations to this MAIC analysis that should be noted while considering its results, many of which are common to all MAICs. Given the absence of IPD for the POLLUX trial, it is challenging to quantify the amount of residual bias in the treatment effect estimates, and it might be possible that some confounding variables may have remained unbalanced. Another drawback of the current study is the limited follow-up data from both clinical studies (median follow-up: IKEMA [20.7 months]; POLLUX [44.3 months for PFS, 13.5 months for OS, and 25.4 months for VGPR or better]); therefore, uncertainties may exist in the current analysis based on the available data. After matching adjustment, there was a substantial reduction in ESS from the original sample size (around 60%, from 112 to 66.2). This reduction reflects that the Isa-Kd population in IKEMA was among other differences with higher cytogenic risk (24% vs. 12% before matching) and more refractory to previous line (35% vs. 28% before matching) than the Dara-Rd treated population in POLLUX, and thus, moderate overlapping with the POLLUX patient population included in this MAIC. In addition, this is unsure whether real-world patients present distributions closest to the POLLUX or IKEMA patient population profiles, and whether this could influence the results as we do not have the POLLUX individual patient data to explore what would be the results with a patient profile closest to IKEMA.
In conclusion, this MAIC demonstrated a significant benefit in PFS, and a statistically non-significant trend was observed for OS and VGPR or better rates in favor of Isa-Kd compared with Dara-Rd. Furthermore, Isa-Kd demonstrated an acceptable safety profile with significantly lower rates of several TEAEs, including febrile neutropenia, compared with Dara-Rd. These results support the value of Isa-Kd as an efficacious treatment option in an early relapse in non-lenalidomide refractory patients compared with Dara-Rd. An updated analysis using extended follow-up data is further warranted to draw definitive conclusions.
AUTHOR CONTRIBUTIONS
Joshua Richter: Conceptualization (equal); investigation (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Peggy L. Lin: Conceptualization (equal); funding acquisition (equal); investigation (equal); project administration (equal); supervision (equal); validation (equal); writing – original draft (equal); writing – review and editing (equal). Viviana Garcia-Horton: Data curation (equal); formal analysis (equal); investigation (equal); resources (equal); software (equal); writing – original draft (equal); writing – review and editing (equal). Patricia Guyot: Conceptualization (equal); investigation (equal); project administration (equal); resources (equal); writing – original draft (equal); writing – review and editing (equal). Erin Singh: Conceptualization (equal); funding acquisition (equal); project administration (equal); resources (equal); supervision (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Zheng-Yi Zhou: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); software (equal); writing – original draft (equal); writing – review and editing (equal). Mark Sievert: Conceptualization (equal); funding acquisition (equal); investigation (equal); project administration (equal); resources (equal); writing – original draft (equal); writing – review and editing (equal). Riley Taiji: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (equal); validation (equal); writing – original draft (equal); writing – review and editing (equal).
ACKNOWLEDGMENTS
The Medical writing support was provided by Pranshu Roy, MPH, and Amit Kandhare from Sanofi.
FUNDING INFORMATION
This study was funded by Sanofi.
CONFLICT OF INTEREST STATEMENT
Joshua Richter reported receiving speaker's bureau fee from Janssen, Celgene, and Adaptive Biotechnologies and is a member of consulting/advisory board of Celgene, Janssen, BMS, Karyopharm, Antengene, Sanofi, X4 Pharmaceuticals, Oncopeptides, Adaptive Biotechnologies, Secura Bio, and Astra Zeneca. Peggy L. Lin, Patricia Guyot, Erin Singh, and Mark Sievert reported being employed by and holding stock in Sanofi. Viviana Garcia-Horton and Zheng-Yi Zhou reported being employed by Analysis Group, which received funding from Sanofi for this research. Riley Taiji was a former employee of Analysis Group, which received funding from Sanofi for this research.
ETHICS APPROVAL STATEMENT
Not applicable. The Declaration of Helsinki and its amendments do not apply to this manuscript, as data used for analyses were completely de-identified (for isatuximab in combination with carfilzomib and dexamethasone [IsaKd]) or abstracted from relevant publications (for daratumumab, in combination with carfilzomib and dexamethasone [DaraRd]). The current study assesses comparative efficacy through statistical analyses and does not involve any clinical intervention. The randomized controlled trials (IKEMA and POLLUX trials), which were the sources of the data used for statistical analyses in the current study, were conducted according to the principles of the Declaration of Helsinki, and its study protocol was approved by ethics committee/IRB for each center with informed consent from participants.
PATIENT CONSENT STATEMENT
Not applicable. The written informed consent is not applicable as this study is a matching-adjusted indirect comparison analysis of individual patient-level data for isatuximab in combination with carfilzomib and dexamethasone obtained from the IKEMA trial (NCT03275285) versus published aggregate data for daratumumab, in combination with carfilzomib and dexamethasone obtained from the POLLUX trial publications.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
CLINICAL TRIAL REGISTRATION
NCT03275285.
PRIOR PRESENTATION
Parts of the data were presented as posters at 18th International Myeloma Workshop, September 8–11, 2021 (Abstract: 1073241). Detailed citation: Richter J, et al. P-213: A matching-adjusted indirect comparison of isatuximab plus carfilzomib and dexamethasone versus daratumumab plus lenalidomide and dexamethasone for relapsed multiple myeloma. Clin Lymphoma Myeloma Leuk. 2021;21(Suppl 2):S156–S157. https://doi.org/10.1016/S2152-2650(21)02340-5. Congress URL: https://www.myelomasociety.org/events/imw-vienna/. 63rd ASH Annual Meeting and Exposition, December 11–14, 2021 (Publication Number: 1962). Detailed citation: Richter J, et al. 1962 A matching-adjusted indirect comparison of isatuximab plus carfilzomib and dexamethasone versus daratumumab plus lenalidomide and dexamethasone for relapsed multiple myeloma. 63rd ASH Annual Meeting and Exposition, December 11–14, 2021; Available from: https://ash.confex.com/ash/2021/webprogram/Paper150299.html. Congress URL: https://www.hematology.org/meetings/annual-meeting.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.