A serum exosomal microRNA-based artificial intelligence diagnostic model for highly accurate detection of hepatocellular carcinoma
Jin-Seong Hwang, Sugi Lee, and Gyeonghwa Kim contributed equally to this work.
Abbreviations
-
- AI
-
- artificial intelligence
-
- AFP
-
- alpha-fetoprotein
-
- AUC
-
- area under the curve
-
- CCl4
-
- carbon tetrachloride
-
- exo-miRNA
-
- exosomal microRNA
-
- HBV
-
- hepatitis B virus
-
- HCV
-
- hepatitis C virus
-
- HCC
-
- hepatocellular carcinoma
-
- miRNA
-
- microRNA
-
- NASH
-
- non-alcoholic steatohepatitis
-
- ND
-
- normal diet
-
- qPCR
-
- quantitative polymerase chain reaction
-
- ROC
-
- receiver operating characteristic
-
- SMOTE
-
- synthetic minority oversampling technique
-
- TCGA
-
- The Cancer Genome Atlas
-
- WD
-
- western diet
Hepatocellular carcinoma (HCC) is a critical cancer worldwide due to its low survival rate [1]. In the United States, the overall 5-year survival rate of patients with HCC is 22%, which decreases sharply with cancer progression [2]. Early detection of HCC improves patient survival. Serum alpha-fetoprotein (AFP) is a widely used biomarker for the diagnosis of HCC, but it is often elevated in patients with cirrhosis, resulting in false-positive results [3]. Diagnostic markers for early detection of HCC have been investigated previously [4], but none are widely applied in clinical settings. HCC pathogenesis is closely associated with hepatitis B and C virus (HBV and HCV) infections, which induce chronic inflammation, leading to cirrhosis and elevating the risk of malignant transformation [5]. Environmental and lifestyle factors, such as diet and alcohol consumption, drive the progression from steatosis to fibrosis, cirrhosis, and eventually HCC [6]. Due to HCC's multifactorial etiology and prolonged progression, identifying early diagnostic biomarkers remains a challenge [7]. Thus, integrating analyses of both pre-HCC and cancerous samples is essential for developing robust early detection strategies. This study aimed to: (1) establish stepwise animal models for HCC-related conditions including non-alcoholic steatohepatitis (NASH) and fibrosis [8]; (2) identify exosomal microRNA (exo-miRNA) signatures for early HCC diagnosis; and (3) develop and validate an artificial intelligence (AI)-based multi-marker model combining exo-miRNAs and AFP levels for accurate HCC diagnosis using clinical samples (Supplementary Figure S1, Supplementary Materials and Methods).
Initially, stepwise mouse models of liver diseases were developed (Figure 1A-C, Supplementary Figure S2A-B). Highly similar gene expression patterns between mouse and human liver diseases were discovered using transcriptome analysis of liver tissues and comparison with public databases (Supplementary Figure S2C). The serum exosomes were then isolated and characterized (Figure 1D, Supplementary Figure S2D), followed by exo-miRNA profiling using Nanostring analysis. This profiling was conducted on mouse models of liver diseases and human samples, which included healthy individuals (n = 7), patients with cirrhosis (n = 6), and patients with HCC (n = 18) (profiling set; Supplementary Table S1).
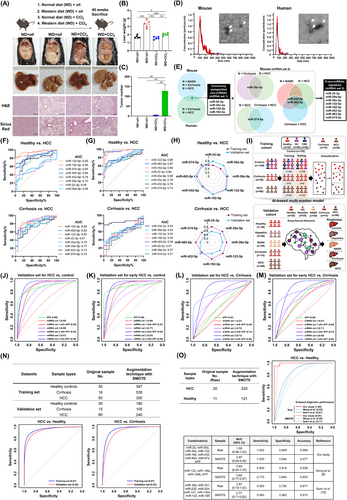
The selection criteria for exo-miRNAs included the upregulation of exo-miRNAs in serum exosomes from mice or humans with liver diseases compared with levels in exosomes from normal mice or healthy controls. Four exo-miRNAs were upregulated in samples from mice or humans with HCC compared with those from normal mice or healthy controls. Subsequently, additional criteria were applied to distinguish between cirrhosis and HCC using the mouse model, resulting in 4 additional exo-miRNAs. Eight exo-miRNAs were finally identified: miR-22-3p, miR-30a-5p, miR-30e-5p, miR-122-5p, miR-192-5p, miR-432-5p, miR-483-5p, and miR-574-5p (Figure 1E, Supplementary Figure S3). These 8 exo-miRNAs were significantly upregulated in the serum exosomes from patients with HCC compared with those from healthy controls, as confirmed by public data analysis (Supplementary Figure S4).
To validate the expression of 8 exo-miRNAs in human serum, quantitative PCR (qPCR) was performed on a training set (n = 195) including healthy controls and patients with cirrhosis and with HCC (training set; Supplementary Table S2). Five exo-miRNAs (miR-22-3p, miR-122-5p, miR-192-5p, miR-483-5p, and miR-574-5p) were significantly upregulated in patients with HCC compared to controls, with exo-miR122-5p showing the highest area under the curve (AUC; 0.95). All exo-miRNAs were significantly elevated in patients with HCC compared to patients with cirrhosis, with AUC values of 0.72-0.88 (Supplementary Figure S5A, Figure 1F). Validation in a new cohort (n = 175; Supplementary Table S3) confirmed that all exo-miRNAs were significantly upregulated in HCC patients compared to healthy controls, with miR-122-5p exhibiting the highest AUC (0.99). Five exo-miRNAs were significantly elevated in patients with HCC compared to patients with hepatitis and cirrhosis (Supplementary Figure S5B-C, Figure 1G). Whereas individual exo-miRNAs demonstrated strong diagnostic potential, combining them into a multi-marker approach was essential to address cohort variability and enhance diagnostic accuracy for HCC (Figure 1H).
To develop a robust diagnostic approach for HCC, AI-based deep-learning was used to construct a multi-marker model incorporating exo-miRNAs and AFP levels (Figure 1I). Prior to model development, batch effect correction was applied to the qPCR data to ensure consistency (Supplementary Figure S6). The model was developed using a training set comprising samples from healthy controls, patients with cirrhosis, HCC, gastric, and colorectal cancer samples (Supplementary Table S2). Diagnostic groups were formed based on AFP levels, 4 exo-miRNAs (miRNA set 1: miR-22-3p, miR-30a-5p, miR-122-5p, and miR-192-5p) with or without AFP levels, 4 mouse-specific exo-miRNAs (miRNA set 2: miR-30e-5p, miR-432-5p, miR-483-5p, and miR-574-5p) with or without AFP levels, and a total of 8 exo-miRNAs (miRNA set 3: combining miRNA set 1 and set 2) with or without AFP levels. The AI model classified the samples into three categories: control, cirrhosis, and HCC. Receiver operating characteristic (ROC) curve analysis indicated that AFP alone could effectively differentiate HCC from gastric and colorectal cancers (AUC > 0.96); combining AFP with miRNA set 1 or set 3 improved performances (AUC = 1.00) (Supplementary Figure S7).
To distinguish healthy controls from patients with HCC, AFP alone achieved moderate diagnostic performance (training set, AUC = 0.94; validation set, AUC = 0.90), whereas miRNA set 1 or set 3 combined with AFP substantially enhanced accuracy (training set, AUC > 0.97; validation set, AUC > 0.95) (Figure 1J, Supplementary Figure S8A, Supplementary Table S4). The miRNA set 3 combined with AFP demonstrated superior diagnostic capability for early-stage HCC (AUC > 0.94), surpassing AFP alone (AUC > 0.89) in both the training and validation sets (Figure 1K, Supplementary Figure S8B, Supplementary Table S5). Using hepatitis or NASH as a control, miRNA set 3 achieved an AUC of 0.96 and 0.94, respectively, which was higher than miRNA set 1 (AUC = 0.73 and 0.69, respectively) (Supplementary Figure S8C). To distinguish cirrhosis from HCC, AFP alone and its combination with miRNA set 1 yielded low performance (validation sets, AUC = 0.57 and 0.49, respectively). However, the miRNA set 3 with AFP achieved high performance (training set, AUC = 1.00; validation set, AUC = 0.90) (Figure 1L, Supplementary Figure S8D, Supplementary Table S6). The miRNA set 3 with AFP consistently outperformed AFP alone for early-stage HCC detection and differentiation from cirrhosis, highlighting its potential as an effective diagnostic tool for HCC (Figure 1M, Supplementary Figure S8E, Supplementary Table S7).
To mitigate overfitting, the synthetic minority oversampling technique (SMOTE) was applied to the miRNA set 3 with AFP model. To distinguish HCC from controls, the AUC values were 0.97 (training) and 0.96 (validation); for cirrhosis versus HCC, the AUC values were 0.97 (training) and 0.93 (validation). These results matched those from the original dataset, demonstrating the model's robustness (Figure 1N, Supplementary Table S8). The miRNA set 3 was compared with 2 previously reported panels [9, 10] using public data that included next-generation sequencing and AFP level (GSE83977). When combined with AFP levels, our miRNA set 3 achieved superior diagnostic accuracy (AUC = 1.00 for Raw data and 0.97 for SMOTE) compared to the three-miRNA panel (AUC = 0.83 for Raw, 0.82 for SMOTE), and the alternative 8-miRNA panel (AUC = 0.87 for Raw, 0.77 for SMOTE). The AI-based, multi-marker model that incorporated 8 exo-miRNAs with AFP demonstrated the highest diagnostic accuracy for HCC across both original and augmented sample sizes (Figure 1O). This superior performance was also observed in the logistic regression-based diagnostic model, where the combination of miRNA set 3 with AFP achieved high diagnostic accuracy (Supplementary Figure S9).
In conclusion, a comprehensive biomarker discovery process was conducted using mouse models of liver disease and human serum samples. The use of mouse models in the diagnostic marker selection process identified valuable markers that might have been excluded when analyzing only human samples. The AI-based, multi-marker model that incorporated a combination of 8 exo-miRNAs with AFP level demonstrated high diagnostic performance, effectively distinguishing between a normal condition and early-stage HCC, and between cirrhosis and HCC. Previous studies have investigated blood-based diagnostics for HCC, highlighting the promise of liquid biopsy techniques [11, 12]. Expanding on this, our AI-based diagnostic model incorporating an exo-miRNA signature exhibits potential as a non-invasive detection tool with high accuracy and can be universally applied to various HCC types. However, to translate these strengths of our study into clinical applications, several challenges need to be addressed through further research. Specifically, (1) simplifying the exosome extraction process, (2) enhancing the AI diagnostic accuracy by incorporating diverse clinical samples from a broader population, and (3) conducting large-scale, multi-center validation studies to enhance the generalizability of the AI-based exo-miRNA diagnostic model across diverse populations.
AUTHOR CONTRIBUTIONS
Jin-Seong Hwang, Sugi Lee, Kiyoon Kwon, Gyeonghwa Kim, Hoibin Jeong, and Hyun-Soo Cho performed the experiments and collected the data. Sugi Lee, Dae-Soo Kim, Tae-Su Han, and Kiyoon Kwon analyzed the bioinformatics data. Eunsun Jung, Yuna Roh, Taesang Son, Hana Lee, Moo-Seung Lee, Kyoung-Jin Oh, Hye Won Lee, Yu Rim Lee, Soo Young Park, Won Young Tak, and Hyun Seung Ban analyzed and interpreted the data. Tae-Su Han, Dae-Soo Kim, Keun Hur, Jang-Seong Kim, and Mi-Young Son drafted and revised the manuscript. Tae-Su Han, Dae-Soo Kim, Keun Hur, Jang-Seong Kim, and Mi-Young Son confirmed the authenticity of all the raw data. All authors have read and approved the final manuscript.
ACKNOWLEDGEMENTS
Not applicable.
FUNDING INFORMATION
This research was supported by grants from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2020R1C1C1007431, NRF-2022R1A2C1003118, RS-2024-00341766, RS-2025-00514590, and NRF-2021R1A5A2021614), the Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korean government (the Ministry of Science and ICT, the Ministry of Health & Welfare, 21A0404L1), and the KRIBB Research Initiative Program.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
ETHIC STATEMENT
This study was approved by the Public Institutional Review Board of the Ministry of Health and Welfare of the Republic of Korea (P01-202105-31-011) and the Ethics Committee of Kyungpook National University Hospital (#KNUH-2014-04-056-001). All patients provided written informed consent prior to sample collection. The animal protocol was approved by the Committee on Animal Experimentation of the Korea Research Institute of Bioscience and Biotechnology (Approval No. KRIBB-AEC-19155).
Open Research
DATA AVAILABILITY STATEMENT
The RNA-seq data (GSE298745) and the Nanostring nCounter data (GSE298588) have been deposited in the NCBI GEO database. All other data supporting the findings of this study are available from the corresponding author upon reasonable request.