Efficacy and safety of KN026 and docetaxel for HER2-positive breast cancer: a phase II clinical trial
Jianli Ma, Jingxuan Wang, and Ting Xu contributed equally to this work
Abstract
Background
The standard first-line treatment for human epidermal growth factor receptor 2 (HER2)-positive recurrent/metastatic breast cancer currently includes pertuzumab plus trastuzumab and docetaxel. This study aimed to evaluate the effectiveness of KN026, an anti-HER2 bispecific antibody, plus docetaxel in first-line treatment of HER2-positive recurrent/metastatic breast cancer.
Methods
This open-label, single-arm, phase II study enrolled patients with HER2-positive recurrent/metastatic breast cancer in 19 centers across China from December 30, 2019 to May 27, 2021. Patients were administered KN026 (30 mg/kg) plus docetaxel (75 mg/m2) in 21-day cycles. Primary endpoints included the objective response rate (ORR) and duration of response (DOR). In addition, overall survival (OS), progression-free survival (PFS), clinical benefit rate (CBR) and safety profile were examined.
Results
A total of 57 patients were included. In the efficacy analysis set of 55 patients, the ORR was 76.4% (95% confidence interval [CI], 63.0%-86.8%), and the CBR was 85.5% (95% CI, 73.3%-93.5%). The median DOR was not reached (95% CI, 20.7 months-not reached). In the safety set of 57 patients, the median PFS was 27.7 months (95% CI, 18.0 months-not reached). The median OS was not reached, with OS rates at 12, 24 and 30 months of 93.0%, 84.1% and 78.5%, respectively. Grade ≥3 treatment-emergent adverse events (AEs) were detected in 36 (63.2%) patients. No deaths were attributed to KN026 or docetaxel.
Conclusion
KN026 plus docetaxel showed promising efficacy and a manageable safety profile in first-line treatment of HER2-positive recurrent/metastatic breast cancer.
List of abbreviations:
-
- AEs
-
- adverse events
-
- CI
-
- confidence interval
-
- CBR
-
- clinical benefit rate
-
- CR
-
- complete response
-
- CSCO
-
- Chinese Society of Clinical Oncology
-
- DOR
-
- duration of response
-
- ECOG
-
- Eastern Cooperative Oncology Group
-
- EAS
-
- efficacy analysis set
-
- HR
-
- hormone receptor
-
- HER2
-
- human epidermal growth factor receptor 2
-
- IHC
-
- immunohistochemistry
-
- NCCN
-
- National Comprehensive Cancer Network
-
- ORR
-
- objective response rate
-
- OS
-
- overall survival
-
- PFS
-
- progression-free survival
-
- PR
-
- partial response
-
- PD
-
- progressive disease
-
- RECIST
-
- Response Evaluation Criteria in Solid Tumors
-
- SD
-
- stable disease
-
- SS
-
- safety set
-
- TEAEs
-
- treatment-emergent adverse events
-
- TRAEs
-
- treatment-related adverse events
1 BACKGROUND
Breast cancer represents the predominant malignancy diagnosed in women globally and remains a principal cause of cancer-induced deaths [1]. Crucially, 15%-20% of breast cancer patients overexpress human epidermal growth factor receptor 2 (HER2) and are defined as HER2-positive [2, 3]. HER2-positive breast cancer tends to be aggressive, with high tendency for recurrence [4].
Per the National Comprehensive Cancer Network (NCCN) Breast Cancer Clinical Practice Guidelines Version 1.2024 [5] and the Chinese Society of Clinical Oncology (CSCO) Breast Cancer Diagnosis and Treatment Guidelines Version 2023 [6], a combination regimen featuring the “dual HER2 blockade” approach of pertuzumab with trastuzumab, alongside taxane chemotherapy, has become the standard first-line treatment for HER2-positive recurrent/metastatic breast cancer. Empirical evidence from several clinical trials corroborates that “dual HER2 blockade” combination can markedly augment the objective response rate (ORR) to 79.0%-80.2%, conferring a substantial prognostic benefit [7-12]. However, despite the significant response benefit of the “dual HER2 blockade”, there is still room for improvement in progression-free survival (PFS), which ranges from 14.5 to 19.6 months [8, 10, 11, 13].
KN026 is a recombinant humanized bispecific antibody capable of dual interaction with the HER2 protein at domains II and IV − sites recognized by pertuzumab and trastuzumab, respectively. Preclinical studies have demonstrated the in-vivo antitumor efficacy of KN026 on par with the joint effects of trastuzumab and pertuzumab in the treatment of HER2-positive breast cancer [13, 14]. Impressively, KN026 neutralizes tumor cells with acquired resistance to the standard dual therapy [14]. A phase I clinical trial revealed an acceptable safety profile and noteworthy anticancer effects of KN026 in patients with HER2-positive metastatic breast cancer who previously had no response to established targeted therapies [15]. These promising results show that KN026 in combination with docetaxel may be a potential new frontline treatment option for HER2-positive recurrent/metastatic breast cancer.
Therefore, the current open-label, multicenter, phase II clinical trial aimed to examine the efficacy and safety of KN026 plus docetaxel in first-line treatment of patients with HER2-positive recurrent/metastatic breast cancer.
2 METHODS
2.1 Study design and patients
The current open-label, single-arm, multicenter phase II trial was conducted across 19 centers in China.
Eligible patients were aged ≥18 years with histologically proven metastatic or locally recurrent, unresectable and incurable HER2-positive breast cancer, defined as either HER2 immunohistochemical (IHC) 3+ status, HER2 gene amplification confirmed via in situ hybridization [16], or a HER2 gene copy number ≥4 as detected by next-generation sequencing. Patients had not received systemic antitumor therapy in the metastatic phase; however, up to 2 lines of systemic endocrine therapy were allowed. Patients had to have one or more measurable lesions at baseline per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, with the stipulation that if only one measurable lesion was present, it should not have undergone prior radiotherapy or should show significant progression post-radiotherapy; adequate organ function and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.
Patient exclusion was performed for any of the following reasons: untreated active brain or meningeal metastases; cardiotoxic events during previous HER2-targeted therapy; a history of other malignancies; uncontrolled comorbidities, including but not limited to active infections, hypertension and cardiac insufficiency; a history of cardiac systolic dysfunction; a history of drug allergies. Eligibility criteria are detailed in the study protocol.
The current trial was granted ethical approval by the medical ethics review committee of Harbin Medical University Cancer Hospital (No. 2019-180). Informed consent was obtained in written form from all participants.
2.2 Treatment
All participants were administered KN026 (Jiangsu Alphamab Biopharmaceuticals, Suzhou, Jiangsu, China) and docetaxel (No. HJ20140086, Sanofi-Aventis Deutschland GmbH, Frankfurt, Hesse, Germany) in 21-day cycles. KN026 was administered intravenously at 30 mg/kg on day 1 of every cycle. At 2 h after KN026 infusion, the patients received docetaxel intravenously at 75 mg/m2. Study treatment was maintained until the occurrence of disease progression per RECIST 1.1 criteria, initiation of a novel antitumor treatment, consent withdrawal by the participant, loss to follow-up, or conclusion of the trial period.
No dose reduction of KN026 was permitted, and its administration would be suspended upon ≥ grade 3 treatment-related toxicity associated with KN026, until the treatment-related toxicity resolves to ≤ grade 1 level. Docetaxel dosage was allowed to be reduced to 55 mg/m2 if intolerable. Detailed guidelines for KN026 and docetaxel dose adjustment, as well as treatment discontinuation criteria in the event of adverse reactions, are comprehensively described in the trial protocol.
2.3 Assessments
Patients underwent baseline tumor imaging assessments using either computed tomography or magnetic resonance imaging scans within 21 days prior to KN026 and docetaxel administration. Subsequent imaging assessments were conducted every 6 weeks following treatment initiation. After the initial 48-week period, the frequency of these assessments was adjusted to every 12 weeks, continuing until certain endpoints were reached: disease progression as defined by RECIST 1.1 criteria, initiation of a novel antitumor treatment, consent withdrawal, loss to follow-up, or trial conclusion. In instances where subjects achieved a complete response (CR) or partial response (PR) per RECIST 1.1 criteria, a follow-up imaging assessment was scheduled after 6 weeks (but not earlier than 4 weeks) to confirm the response.
Adverse events (AEs) were followed up at each treatment cycle, at the end of treatment and 30 days after the last dose or initiation of a new antitumor therapy.
2.4 Study endpoints
The primary endpoints of this study were investigator-assessed ORR and duration of response (DOR) per RECIST 1.1 criteria. ORR was the rate of patients achieving either a CR or PR. DOR was the period between initial assessment of CR or PR (whichever was first recorded) and any recorded progressive disease (PD) or death from any cause (whichever occurred first) in patients with CR or PR.
Secondary endpoints comprised PFS, alongside 6-month and 12-month PFS rates, clinical benefit rate (CBR), overall survival (OS), 6-month and 12-month OS rates and AEs. PFS was the time between the first dosing of the study drugs and PD onset or death, while CBR was the percentage of patients showing measurable disease at baseline and exhibiting a CR, PR, or stable disease (SD) as best overall response for a duration of ≥24 weeks. Disease progression was evaluated per RECIST 1.1 criteria. OS was the time from the initial dosing of study drugs to death from any cause. The frequencies and severity of AEs were recorded, encompassing treatment-emergent AEs (TEAEs), treatment-related AEs (TRAEs), serious AEs (SAEs), AEs leading to treatment discontinuation, AEs leading to death, and AEs of special interest. AEs of special interest included all cardiac adverse events and non-infectious pneumonitis, regardless of their relation to KN026, observed during the administration period and up to 30 days after the final dose or start of a new antitumor treatment. The severity of these AEs was assessed using National Cancer Institute Common Terminology Criteria for Adverse Events 5.0.
2.5 Statistical analysis
The sample size calculation, anchored on a 95% confidence interval (CI) for ORR, was assessed by the Clopper-Pearson method. Based on varying ORRs and corresponding 95% CIs, different numbers of patients achieving response were estimated, ranging from 20 patients with an ORR of 40% (95% CI: 26.4%-54.8%) to 37 patients when an ORR of 74% (95% CI: 59.7%-85.4%) was considered. Taking into account those possibilities, the projected enrollment was expected to include 50-60 subjects.
The efficacy analysis set (EAS), which included all subjects administered one or more full or partial doses of KN026 or docetaxel with one or more post-treatment efficacy assessments, was utilized for analyzing tumor efficacy endpoints (ORR, CBR and DOR). The safety set (SS), including all subjects administered one or more full or partial doses of KN026 or docetaxel, was employed for safety analyses. Subgroup analyses of efficacy endpoints were conducted based on baseline characteristics such as visceral metastasis, brain metastasis, HER2 IHC findings, tumor stage, and hormone receptor (HR) status. The 95% CIs of ORR and CBR were determined by the Clopper-Pearson method, while median DOR, PFS, and OS and the respective 95% CIs were derived from Kaplan-Meier curves. Further details on the principles for handling missing data are outlined in the study protocol.
Statistical analysis utilized SAS 9.4 (SAS Institute, Cary, NC, USA). Continuous data are presented as median and range, and categorical data as frequency and percentage.
3 RESULTS
3.1 Baseline characteristics of patients
From January 13, 2020 to May 27, 2021, 57 patients with HER2-positive recurrent/metastatic breast cancer were enrolled. They were all treated with KN026 combined with docetaxel. By September 15, 2023, treatment was ongoing in 22 patients, while 35 discontinued treatment. The SS comprised 57 patients, while 55 patients were included in the EAS after completing at least one efficacy evaluation (Figure 1).
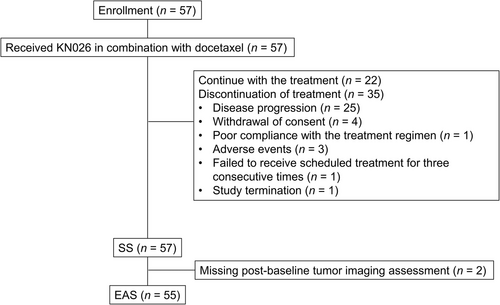
Flowchart of HER2-positive recurrent/metastatic breast cancer patients administered KN026 and docetaxel in this phase II clinical trial.
Abbreviations: HER2, human epidermal growth factor receptor 2; SS, safety set; EAS, efficacy analysis set.
Baseline clinicodemographic features are shown in Table 1. All patients were women with a median age of 52 years. Most patients were at an advanced clinical stage at baseline, including 52 (91.2%) with stage IV disease. HER2 IHC results were predominantly IHC 3+ (84.2%), and HR positivity was found in 26 patients (45.6%). Visceral metastases were detected in 34 patients (59.6%). Of the 37 patients who previously underwent surgical treatment, 2 (3.5%) had palliative surgery, 32 (56.1%) had curative surgery, and 12 (21.1%) had other types of surgery. Of the 12 patients previously administered radiotherapy, 2 (3.5%) had three-dimensional conformal radiotherapy, and 3 (5.3%) underwent intensity-modulated radiation therapy. Adjuvant therapy was previously administered to 30 (52.6%) patients, and neoadjuvant therapy to 5 (8.8%). Previous endocrine therapy was administered to 12 (12.1%) patients, targeted therapy to 4 (7.0%), and chemotherapy to 31 (54.4%); the most commonly employed chemotherapeutic agents included cyclophosphamide (29 cases, 50.9%), docetaxel (23 cases, 40.4%), and epirubicin (14 cases, 24.6%).
| Variable | Whole cohort |
|---|---|
| Total, n | 57 |
| Age, years, median (range) | 52 (30-67) |
| Sex, n (%) | |
| Female | 57 (100.0) |
| Male | 0 |
| Age, n (%) | |
| <65 years | 55 (96.5) |
| ≥65 years | 2 (3.5) |
| ECOG performance status score, n (%) | |
| 0 | 22 (38.6) |
| 1 | 35 (61.4) |
| Clinical stage at baselinea, n (%) | |
| IIIA | 1 (1.8) |
| IIIB | 2 (3.5) |
| IIIC | 2 (3.5) |
| IV | 52 (91.2) |
| HER2 status, assessed by IHC, n (%) | |
| IHC1+ | 1 (1.8) |
| IHC2+ | 8 (14.0) |
| IHC3+ | 48 (84.2) |
| HER2 status, assessed by ISH, n (%) | |
| Positive | 13 (22.8) |
| Negative | 0 |
| Data not available | 44 (77.2) |
| Hormone receptor status, n (%) | |
| ER-positive and/or PR-positive | 26 (45.6) |
| ER-negative and PR-negative | 24 (42.1) |
| Unknown | 7 (12.3) |
| Recurrent disease, n (%) | 29 (50.9) |
| Metastatic status at baseline, n (%) | |
| Absent | 5 (8.8) |
| Non-visceral | 23 (40.4) |
| Brain | 6 (10.5) |
| Bone | 24 (42.1) |
| Lymph node | 40 (70.2) |
| Pleura | 14 (24.6) |
| Others | 22 (38.6) |
| Visceral | 34 (59.6) |
| Lung | 24 (42.1) |
| Liver | 22 (38.6) |
| Adrenal gland | 2 (3.5) |
| Ovary | 1 (1.8) |
| Prior surgery, n (%) | 37 (64.9) |
| Adjuvant or neoadjuvant therapy, n (%) | 35 (61.4) |
| Prior radiotherapy, n (%) | 12 (21.1) |
| Prior endocrine therapy, n (%) | 12 (21.1) |
| Prior chemotherapy, n (%) | 31 (54.4) |
| Cyclophosphamide | 29 (50.9) |
| Docetaxel | 23 (40.4) |
| Epirubicin | 14 (24.6) |
| Prior targeted therapy (Trastuzumab), n (%) | 4 (7.0) |
- Abbreviations: HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization; ER, estrogen receptor; PR, progesterone receptor; ECOG, Eastern Cooperative Oncology Group.
- a Baseline clinical tumor stage was determined per the 8th edition of the American Joint Committee on Cancer (AJCC) staging systems for breast cancer.
3.2 Tumor response
The median time of exposure to study drugs was 674 (range, 21-1,171) days, and the median duration of treatment was 28 (range, 1-52) weeks. Totally 49 (86.0%) patients in the SS underwent more than six cycles of therapy.
In the EAS comprising 55 patients, 3 patients achieved CR and 39 achieved PR, culminating in an ORR of 76.4% (95% CI, 63.0%-86.8%). The CBR was 85.5% (95% CI, 73.3%-93.5%) (Table 2). Subgroup analyses of ORR stratified by baseline visceral and brain metastases, HER2 IHC findings, clinical stage, and HR status revealed that the therapeutic benefits extended across most subgroups, maintaining a consistent trend with the total population (Supplementary Figure S1).
| Response | SS [n (%)] |
|---|---|
| Total | 55 |
| Best overall response, n (%) | |
| CR | 3 (5.5) |
| PR | 39 (70.9) |
| SD | 13 (23.6) |
| SD ≥24 weeks | 5 (9.1) |
| PD | 0 |
| NE | 0 |
| ORR, n (%; 95% CI) | 42 (76.4; 63.0-86.8) |
| CBR, n (%; 95% CI) | 47 (85.5; 73.3-93.5) |
| DOR, months, median (95% CI) | NR (20.7, NR) |
- Abbreviations: SS, safety set; ORR, objective response rate; CBR, clinical benefit rate; DOR, duration of response; CI, confidence interval; IHC, immunohistochemistry; HR, hormone receptor; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NR, not reached.
In addition, the median DOR was not reached (95% CI, 20.7 months-not reached) in the 42 responding patients (Table 2). DOR rates at 12, 24 and 30 months were 85.2%, 59.3% and 51.6%, respectively (Figure 2A).
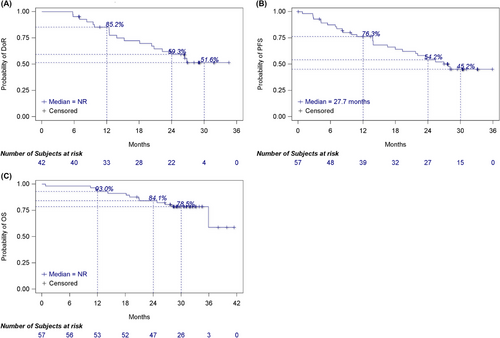
Kaplan-Meier survival curves for A) DOR, B) PFS, and C) OS in recurrent/metastatic breast cancer patients who received KN026 and docetaxel.
Abbreviations: NR, not reached; DOR, duration of response; PFS, progression-free survival; OS, overall survival.
3.3 Survival
Median follow-up was 31.1 months (range: 0.8-41.4 months), and median PFS was 27.7 months (95% CI, 18.0 months-not reached) (Figure 2B). PFS rates at 6, 12, 24 and 30 months were 87.4% (95% CI, 75.4%-93.8%), 76.3% (95% CI, 62.6%-85.5%), 54.2% (95% CI, 39.7%-66.6%), and 45.2% (95% CI, 30.9%-58.4%), respectively (Figure 2B). Subgroup analyses of PFS stratified by baseline visceral and brain metastases, HER2 IHC findings, clinical stage, and HR status indicated survival benefits across most groups, consistent with the overall cohort (Supplementary Figure S2).
Median OS was not reached, and OS rates at 6, 12, 24, and 30 months were 98.2% (95% CI, 88.2%-99.8%), 93.0% (95% CI, 82.4%-97.3%), 84.1% (95% CI, 71.7%-91.4%), and 78.5% (95% CI, 65.2%-87.2%), respectively (Figure 2C).
3.4 Safety profiles
All patients experienced TEAEs. Grade ≥3 TEAEs were recorded in 36 patients (63.2%). SAEs were documented in 12 patients (21.1%), of whom 10 (17.5%) had an SAE of grade ≥3 (Supplementary Table S1). Eleven patients died due to disease progression. No deaths were attributed to KN026 or docetaxel. A fatal AE of unknown cause was reported in 1 patient who received one dose of KN026. In this patient, KN026 administration was stopped at 12 min with 162.8 mg (planned dose: 1,860 mg) due to grade 3 allergic reactions, which were resolved on the same day at discharge. The patient was withdrawn from the study without the use of docetaxel and ten days later developed grade 4 sepsis. The patient died more than 23 days after KN026 administration, and this death was considered not related to either KN026 or docetaxel.
The commonest TEAEs (occurring in ≥20% of patients) were white blood cell count decrease (63.2%), neutrophil count decrease (61.4%) and diarrhea (36.8%). The commonest grade ≥3 TEAEs (≥2%) included neutrophil count decrease (40.4%), white blood cell count decrease (28.1%) and hypokalemia (8.8%) (Table 3).
| EAS [n (%)] | ||
|---|---|---|
| Event | Any grade | Grade ≥3 |
| White blood cell level decrease | 36 (63.2) | 16 (28.1) |
| Neutrophil level decrease | 35 (61.4) | 23 (40.4) |
| Diarrhea | 21 (36.8) | 2 (3.5) |
| Alanine aminotransferase increase | 20 (35.1) | 0 |
| Aspartate aminotransferase increase | 20 (35.1) | 0 |
| Anemia | 18 (31.6) | 1 (1.8) |
| Alopecia | 13 (22.8) | 0 |
| Hypokalemia | 12 (21.1) | 5 (8.8) |
| Infusion related reaction | 11 (19.3) | 0 |
| Weight decrease | 11 (19.3) | 1 (1.8) |
| Pyrexia | 7 (12.3) | 0 |
| Urinary tract infection | 6 (10.5) | 0 |
| Vomiting | 6 (10.5) | 1 (1.8) |
| Blood bilirubin increase | 6 (10.5) | 0 |
| Lymphocyte count decrease | 5 (8.8) | 2 (3.5) |
| Hypocalcemia | 4 (7.0) | 2 (3.5) |
| Febrile neutropenia | 3 (5.3) | 3 (5.3) |
| Myelosuppression | 3 (5.3) | 2 (3.5) |
- Abbreviations: TEAEs, treatment-emergent adverse events; EAS, efficacy analysis set.
Fifteen patients (26.3%) had TEAEs leading to KN026 dose interruption and 3 (5.3%) had TEAEs leading to permanent KN026 discontinuation, including one case of grade 3 type I hypersensitivity related to KN026, one grade 3 hypersensitivity related to KN026, and one grade 3 pericardial effusion not related to KN026 and possibly related to disease progression. In addition, 6 patients (10.5%) had docetaxel dose reduction due to a TEAE. Nine patients (15.8%) had TEAEs leading to docetaxel dose interruption, and 2 (3.5%) had TEAEs resulting in permanent discontinuation of docetaxel (Supplementary Table S1).
AEs of special interest were recorded in 5 patients (8.8%). Of these, 3 (5.3%) had grade 2 pneumonitis, and 2 (3.5%) had left ventricular ejection fraction (LVEF) decrease ≥15% from the baseline absolute value, both of which were grade 2 (Supplementary Table S1). There were no reports of symptomatic left ventricular systolic dysfunction or LVEF reduction ≥10% from the baseline absolute value and LVEF <50.
4 DISCUSSION
In this study, the combination of KN026 with docetaxel was evaluated in first-line treatment of HER2-positive recurrent/metastatic breast cancer. The results revealed a confirmed ORR of 76.4%, while the median DOR was not reached. Median PFS was 27.7 months and median OS was immature, with OS rates at 12, 24 and 30 months of 93.0%, 84.1% and 78.5%, respectively. In addition, the combination therapy demonstrated a controllable safety profile. These findings demonstrate the potential of KN026 plus docetaxel as a new therapeutic option for patients with HER2-positive recurrent/metastatic breast cancer.
The above finding of an ORR of 76.4% demonstrates a noteworthy similarity with previous landmark studies. In the CLEOPATRA study, the ORR for the pertuzumab, docetaxel, and trastuzumab combination was reported at 80.2% [10]. Similarly, the PERUSE and PUFFIN studies both reported ORRs of 79% for their respective pertuzumab, trastuzumab, and docetaxel regimens [11-13]. Regarding the CBR, our value of 85.5% corroborates a previous trial, which reported a CBR approximating 86% [12]. This suggests that KN026 has response advantages consistent with dual HER2 blockade regimens in HER2-positive recurrent/metastatic breast cancer. Additionally, while the final results of the PERUSE study indicated a median DOR of 20.0 months [12], the current study revealed an immature median DOR with a lower limit of the 95% CI at 20.7 months, with ongoing follow-up.
In terms of survival benefits, the PFS and OS outcomes in this study provide a valuable perspective compared to established therapies in landmark trials. For instance, the phase III CLEOPATRA study reported significantly prolonged median PFS in patients receiving pertuzumab plus docetaxel and trastuzumab (18.5 months) compared with those administered docetaxel plus trastuzumab (12.4 months, P < 0.001) for first-line HER2-positive metastatic breast cancer [10]. OS analysis revealed the median OS was 56.5 months, while OS rates at 12, 36 and 48 months were 94.4%, 68.2% and 57.6%, respectively [9]. In the PERUSE study, a dual HER2 blockade therapy combined with docetaxel achieved median PFS and OS of 19.4 and 66.5 months, respectively [12]. Similarly, the final analysis of the PUFFIN trial showed a median PFS of 16.5 months for the same regimen, with median OS not reached [17]. Our study found a median PFS of 27.7 months, with median OS not reached, and 12-month and 30-month OS rates of 93.0% and 78.5%, respectively. These results align well with the aforementioned studies. Moreover, subgroup analyses in the present study revealed survival benefits in most categories were consistent with the overall benefits. This consistency across studies suggests that KN026 in combination with docetaxel may be effective in first-line treatment for patients with HER2-positive recurrent/metastatic breast cancer, improving patient survival.
It is worth noting that compared with the CLEOPATRA [10], PERUSE [12], and PUFFIN studies [17], PFS in our study was numerically longer (27.7 months). We speculate that this possibly might be due to KN026's ability to simultaneously bind two non-overlapping epitopes of HER2, resulting in HER2 signaling blockade and subsequent tumor inhibition [14]. However, given the heterogeneity of baseline characteristics among patients in our study versus the CLEOPATRA [10], PERUSE [12], and PUFFIN studies [17], the current data should be interpreted with caution as this was a single-arm study with relatively small sample size and direct comparisons between studies are not possible. Future head-to-head studies should compare the different regimens for efficacy and safety. Moreover, the manageable safety profile of KN026 may also have contributed to the PFS benefit in this trial.
The safety profile determined in the current study corroborates previous studies. Grade ≥3 TEAEs were detected in 63.2% of patients. Notably, this study did not identify any additional safety signals, aligning with the established safety profiles of dual HER2 blockade therapies [7-13]. In addition, AEs resulted in discontinued pertuzumab and trastuzumab administration in 10% and 9% of patients in the PERUSE study, respectively [12], whereas KN026 administration was discontinued in 5.3% of patients due to AEs in this study. Regarding cardiac toxicity, left ventricular systolic dysfunction of any grade was detected in 4.4% of the pertuzumab arm in the CLEOPATRA study, with grade 3 or higher found in 1.2% [10]. In contrast, the current study reported only two cases (3.5%) of grade 2 LVEF decrease, with no cases of symptomatic left ventricular systolic dysfunction or LVEF decreases below 50% from the baseline absolute value and no grade ≥3 cardiac toxic events. In PERUSE, 90 patients (6%) experienced an LVEF decline [12]. The PUFFIN study recorded 2 patients (1.7%) in the pertuzumab group (n = 122) with LVEF reductions ≥10% from baseline and absolute values <50% [13]. In summary, the safety profile of KN026 combined with docetaxel in this study was good and manageable, without novel safety concerns. This finding suggests that KN026 provides a safety advantage, particularly in terms of cardiac toxicity.
However, the present study had limitations. First, a small sample size was used, which might limit the generalizability of findings. Additionally, the absence of a control group in the study design restricts the ability to make direct comparative assessments against standard therapies. Furthermore, this study lacked long-term follow-up, which is crucial for assessing long-term benefits, particularly in OS. To address these shortcomings and build upon these preliminary findings, we are planning a more extensive, randomized, controlled phase III clinical trial (ClinicalTrials.gov, registration number: NCT05838066).
5 CONCLUSIONS
The combination of KN026 with docetaxel demonstrates promising efficacy and safety in first-line treatment of HER2-positive recurrent/metastatic breast cancer. Subsequent large, randomized controlled studies are warranted to validate the clinical benefits of KN026 plus docetaxel.
AUTHOR CONTRIBUTIONS
Conception and design: Jianli Ma, Jingxuan Wang, Ting Xu, Qingyuan Zhang. Provision of study materials or patients: Jianli Ma, Jingxuan Wang, Quchang Ouyang, Xiaojia Wang, Jingfen Wang, Lu gan, Zhong Ouyang, Daren Lin, Tao Sun, Changping Shan, Herui Yao, Baochun Zhang, Zhengguang Li, Zhixiang Zhuang, Ying Lu, Hongwei Yang, Jian Huang, Xingwang Yang, Hongmei Sun. Collection and assembly of data: Jianli Ma, Jingxuan Wang, Ting Xu. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
ACKNOWLEDGMENTS
We thank the patients, their families, the investigators, and the teams who participated in this study.
FUNDING STATEMENT
None.
CONFLICT OF INTEREST STATEMENT
Ting Xu is an employee of Jiangsu Alphamab Biopharmaceuticals Co., Ltd. Other authors declare that they have no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was granted ethical approval by the medical ethics review committee of Harbin Medical University Cancer Hospital (approval number: 2019-180).
Informed consent was obtained in written form from all participants.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov Identifier: NCT04165993.
TRIAL REGISTRATION
ClinicalTrials.gov Identifier: NCT04165993
Open Research
DATA AVAILABILITY STATEMENT
All data are included in the article or uploaded in the Supplementary Appendix. Aggregated clinical data are available upon request to the corresponding author (Q.Z) provided that there is no reasonable risk of de-anonymizing study participants. Individual patient data cannot be shared due to privacy restrictions.




