Efficient 5-ALA-photodynamic therapy in nasopharyngeal carcinoma induces an immunoactivation mediated by tumoral extracellular vesicles and associated with immunogenic cell death
Nadira Delhem and Olivier Moralès contributed equally to this work.
Abbreviations
-
- 5-ALA
-
- 5-aminolevulinic acid
-
- BL
-
- B Lymphocyte
-
- CM
-
- Conditioned Media
-
- CRT
-
- Calreticulin
-
- Ct
-
- Cycle Threshold
-
- DAMPS
-
- Damage Associated Molecular Patterns
-
- DC
-
- Dendritic Cell
-
- DCF
-
- 2',7'-dichlorofluorescein
-
- DCFDA
-
- 2',7'-dichlorofluorescein diacetate
-
- dsDNA
-
- Double-stranded DNA
-
- eATP
-
- Extracellular ATP
-
- ELISA
-
- Enzyme-Linked Immunosorbent Assay
-
- EV
-
- Extracellular Vesicles
-
- H2O2
-
- Hydrogen peroxide
-
- HKG
-
- Housekeeping Genes
-
- HMGB1
-
- High Mobility Group Box 1
-
- HSP
-
- Heat Shock Protein
-
- Illu
-
- Illumination Only
-
- MFI
-
- Median Fluorescence Intensity
-
- NK
-
- Natural Killer
-
- NPC
-
- Nasopharyngeal Carcinoma
-
- NT
-
- Non-treated
-
- PBMC
-
- Peripheral Blood Mononuclear Cell
-
- PBS
-
- phosphate-buffered saline
-
- PDT
-
- Photodynamic Therapy
-
- PpIX
-
- Protoporphyrin IX
-
- PS
-
- Photosensitizer
-
- R/M
-
- Recurrence and Metastasis
-
- RFU
-
- Relative Fluorescence Unit
-
- ROS
-
- Reactive Oxygen Species
-
- RT
-
- Room Temperature
-
- RT-QPCR
-
- Real-Time Quantification Polymerase Chain Reaction Assays
-
- SEV
-
- Small Extracellular Vesicle
-
- TL
-
- T Lymphocyte
-
- Treg
-
- Regulatory T cell
-
- TRPS
-
- Tunable Resistive Pulse Sensing
Nasopharyngeal carcinoma (NPC) is a rare cancer, with 120,334 cases worldwide in 2022, but it remains endemic in Southeast Asia and North Africa. Early-stage NPC is typically treated with radiotherapy, often combined with chemotherapy for advanced stages [1]. Despite a 5-year survival rate of 70% to 90% for locoregional disease, late-stage diagnosis and locoregional or distant recurrence and metastasis (R/M) result in a poor prognosis for many patients, underscoring the urgent need for novel therapeutic strategies [2-4]. The tumor microenvironment, enriched with immunosuppressive elements such as M2 macrophages, myeloid-derived suppressor cells (MDSCs), and regulatory T cells (Tregs), plays a central role in promoting immune evasion and resistance to therapy. In NPC, effective therapeutic strategies should not only induce tumor cell death but also reprogram this immunosuppressive microenvironment to restore robust anti-tumor immunity. To address these challenges, we propose evaluating the efficacy of photodynamic therapy (PDT) and its immunoactivating properties in NPC. PDT is a non-invasive treatment that induces cell death via reactive oxygen species (ROS) and activates an anti-tumor immune response by releasing tumor antigens and damage-associated molecular patterns (DAMPs) [5, 6]. After examining the direct cell death induced by 5-aminolevulinic acid (5-ALA)-PDT, we investigated its ability to trigger immune activation and its effects on immune cell populations and their secretome. Lastly, we conducted an in-depth analysis of molecular and vesicular (extracellular vesicle) components to understand the mechanisms underlying immune response activation. Detailed study designs and methods are available in the Supplementary Materials.
The prodrug 5-ALA is preferentially absorbed by tumor cells and metabolized into protoporphyrin IX (PpIX), the photosensitizer, via the heme synthesis pathway (Supplementary Figure S1A). To evaluate 5-ALA-PDT in NPC cell lines, we assessed their capacity to convert 5-ALA into PpIX. We observed that NPC cell lines expressed key enzymes and transporters involved in the heme pathway, with no significant differences, confirming their ability to metabolize 5-ALA into PpIX (Supplementary Figure S1B). We then incubated NPC cells with varying concentrations of 5-ALA, showing successful conversion of 5-ALA into intracellular PpIX after 2 hours, followed by extracellular release of PpIX between 6 to 8 hours (Figure 1A, Supplementary Figure S1C). Based on these findings and considering clinical data, we selected a 4-hour incubation period for subsequent experiments.
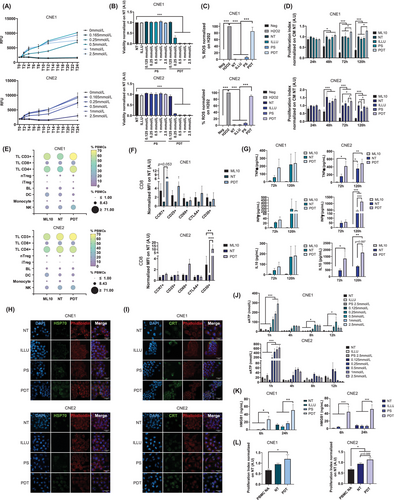
To determine the direct impact and efficacy of 5-ALA-PDT on NPC cell lines, we subjected the cells to 5-ALA-PDT. A dose-dependent reduction in cell viability was observed, with CNE2 cells (EC50: 104.9 µmol/L) exhibiting greater sensitivity to treatment compared to CNE1 cells (EC50: 209.7 µmol/L) (Figure 1B, Supplementary Figure S1D). No cell toxicity was observed under non-PDT conditions. Moreover, as expected, a significant release of ROS was detected in the PDT condition, with an average positivity rate of 85% (CNE1) and 89% (CNE2) (Figure 1C, Supplementary Figure S1E).
Having demonstrated the direct effect of 5-ALA-PDT, we investigated the type of cell death induced, focusing on apoptosis and necrosis (Supplementary Figure S2A-B). Predominantly necrotic cell death was observed, with a dose-dependent increase peaking at 0.5 mmol/L for CNE1 and 1 mmol/L for CNE2 (Supplementary Figure S3A-B). Next, we measured the secretion of various cytokines in the supernatants of treated and untreated cells to assess whether tumor cells release cytokines upon treatment (Supplementary Figure S2C). In CNE1 cells, IL-6 secretion decreased during the early post-PDT period, then increased at 24 hours, while in CNE2 cells, a prolonged decrease lasting up to 72 hours post-PDT was observed. For TGF-β, a decrease in secretion was noted starting at 48 hours post-5-ALA-PDT in CNE1, with no significant change in CNE2.
5-ALA-PDT has been observed to induce cell death, predominantly necrotic, and to modulate the tumor cell secretome, which may indicate a potential immunoactivating effect of this therapeutic modality. To explore this, we investigated the effect of conditioned media (CM) collected 24 hours post-5-ALA-PDT at EC80 on immune cell proliferation. For the CNE1 cell line, CM-PDT significantly increased the proliferation of non-activated peripheral blood mononuclear cell (PBMC) at 72 hours (23%) and 120 hours (28%) compared to non-treated (NT) CM (CM-NT). Similarly, for the CNE2 cell line, proliferation increased by 27% at 72 hours, demonstrating an immunoactivating effect of CM-PDT across both cell lines (Figure 1D).
Given the proliferative effects of post-5-ALA-PDT CM on PBMCs, we further examined its impact on different immune cell populations. For the CNE1 cell line, at 72 hours post-culture, we observed an increase in the prevalence of CD3+ T lymphocytes (TLs) (50.46% vs. 70.63%), supported by an increase in CD4+ TLs (42.8% vs. 63.36%) and CD8+ TLs (14.10% vs. 18.06%), as well as in natural killer cells (NK) (17.8% vs. 28.36%) when PBMCs were cultured with CM-PDT compared to CM-NT. In parallel, we observed a decrease in the prevalence of B Lymphocyte (BL) (8.27% vs. 2.83%) and dendritic cell (DC) (13.01% vs. 6.13%) (Figure 1E). A slight increase in induced regulatory TL (iTreg) was observed at 72 hours (0.2% vs. 1.63%), which was offset by a decrease at 120 hours (Figure 1E, Supplementary Figure S4A-B). For the CNE2 cell line, there also appeared to be an increase in the prevalence of CD3+ TLs (66.5% vs 69.66%) when PBMCs were cultured with CM-PDT compared to CM-NT at 72 hours (Figure 1E).
Following the observed impact of CM-PDT on lymphocyte populations, we analyzed their activation status using specific markers at the same time points. For CNE1, there was an increase in CD8+CCR7+ memory TLs at 72 hours (Figure 1F) and in CD4+CCR7+ memory TLs at 120 hours when PBMCs were cultured with CM-PDT compared to CM-NT (Supplementary Figure S4C). For CNE2, we observed an increase in CD8+CD30+ late-activated TLs at 72 hours (Figure 1F) and CD8+CCR7+ memory T cells at 120 hours (Supplementary Figure S4D). Additional markers, such as CD69+ and CD25+, also appeared elevated in the CD8+ population, although these increases were not statistically significant. Moreover, at 120 hours, we noted an increase in CD4+CCR7+ memory TLs and a decrease in CD4+CD69+ early-activated TLs (Supplementary Figure S4D, Supplementary Figure S5).
As another indicator of immune activation, we analyzed the PBMC cytokine secretome, focusing on a range of cytokines (Supplementary Figure S6). When non-activated PBMCs were treated with CM-PDT, there was a trend toward increased secretion of anti-tumoral cytokine, IFN-γ and TNF-α, along with an increase in the immunoregulatory cytokine IL-10 (Figure 1G). These results suggest that CM-PDT exhibits immunoactivating properties, promoting a Th1-type response with enhanced lymphocyte activation markers and anti-tumor cytokine release.
To evaluate the immunogenic potential of 5-ALA-PDT-induced cell death, we measured the release of DAMPs by NPC-treated cells. First, we examined calreticulin (CRT) and heat shock protein 70 (HSP70) exposure using confocal microscopy. Although some signals were observed in control cells, a significant increase in CRT and HSP70 exposure was noted under PDT conditions, with more intense and homogeneous signal (Figure 1H-I, Supplementary Figure S7). Next, we observed increased extracellular ATP (eATP) release 1-hour post-5-ALA-PDT, which was more pronounced in CNE2 cells (Figure 1J), and increased extracellular High Mobility Group Box 1 (HMGB1) secretion compared to controls (Figure 1K). These findings suggest that the release/exposure of DAMPs by NPC cells following 5-ALA-PDT may contribute to immune response activation via the CM.
A key player in the tumor microenvironment is tumor-derived exosomes, which potentiate the suppressive activity and recruitment of Treg [7] and induce alterations in DC maturation, favoring a regulatory phenotype [8]. We isolated small extracellular vesicles (SEVs) from the CM-NT and CM-PDT of cells treated with 5-ALA-PDT at EC80, characterizing them based on size and protein content, which suggested they are exosomal vesicles (Supplementary Figure S8A-C). Further investigation showed that PDT-SEV induced a significant increase in PBMC proliferation (78.6%) compared to non-activated PBMCs for CNE1. Similarly, for CNE2, PDT-SEV induced proliferation compared to both non-activated PBMCs (68.65%) and NT-SEV controls (21%) (Figure 1L). Thus, PDT appears to modify the functionality of SEVs', potentially contributing to immune activation and proliferation. A more detailed examination of the vesicular content revealed an increase in double-stranded DNA (dsDNA) in SEVs following treatment. Given that dsDNA has the potential to activate recognition receptors such as Toll-like receptor 9 (TLR-9) or the cGAS/STING pathway, it is plausible that these mechanisms contribute to the immunoactivating effect observed from exosomes (Supplementary Figure S8D).
In conclusion, we demonstrated for the first time that 5-ALA-PDT induces necrotic tumor cell death in NPC cell lines, accompanied by ROS release, and may promote a Th1-type immune response. This response is marked by lymphocyte proliferation and activation, along with the release of pro-inflammatory cytokines, potentially triggered by the release/exposure of DAMPs and the secretion of immunostimulatory extracellular vesicles. These findings suggest that 5-ALA-PDT could be a promising adjuvant therapy for R/M NPC patients, alongside conventional treatments, by inducing tumor cell death and promoting a longer-term anti-tumor immune response. Therefore, combining 5-ALA-PDT with immunotherapy, especially immune checkpoint inhibitors, may offer a synergistic effect for patients with limited treatment options.
AUTHOR CONTRIBUTIONS
Camille Trioën, Thomas Soulier, Jacquie Massoud, Clément Bouchez, and Nicolas Stoup performed all experiments. Camille Trioën performed data analysis and finalization. Camille Trioën, Anthony Lefebvre, Jacquie Massoud, Guillaume Paul Grolez, Anne-Sophie Dewalle, and Olivier Morales established the methodology. Camille Trioën and Olivier Morales drafted and revised the manuscript. Nadira Delhem and Olivier Morales supervised this study and revised the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
The authors would like to thank the BioImaging Center Lille for providing access to flow cytometry and confocal microscopy equipment.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
Camille Trioën was funded by a grant from la Région des Hauts-de-France and the university of Lille.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Human blood samples were collected from healthy adult donors (EFS PLER/2021/005) with informed consent obtained in accordance with approval of the Institutional Review Board of French Ministry of Research and Higher Education (DC-2020-3942).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




