Training the synergy between Bacillus Calmette-Guérin and immune checkpoint-blocking antibodies in bladder cancer
List of abbreviations
-
- BCG
-
- Bacillus Calmette-Guérin
-
- NMIBC
-
- Non-muscle invasive bladder cancer
-
- EAU
-
- European Association of Urology
-
- RC
-
- Radical cystectomy
-
- TNF
-
- Tumor necrosis factor
-
- IL
-
- Interleukin
-
- FcR
-
- Fc Receptor
-
- NOD2
-
- Nucleotide-binding oligomerization domain-containing protein 2
-
- TLR
-
- Toll-like Receptor
-
- Akt/mTOR
-
- Protein kinase B/mammalian Target of Rapamycin
-
- IgG
-
- Immunoglobulin G
-
- PD-1
-
- Programmed Cell Death Protein 1
-
- PD-L1
-
- Programmed Cell Death Ligand 1
-
- TAM
-
- Tumor-associated Macrophage
Fifty years after the introduction of Bacillus Calmette-Guérin (BCG), a live attenuated strain of Mycobacterium bovis [1], it is still the most effective and successful adjuvant immunotherapy of non-muscle invasive bladder cancer (NMIBC) [2]. The guidelines of the European Association of Urology (EAU) suggest a 6-weekly induction phase followed by a maintenance schedule of BCG once weekly for 3 weeks and at 3, 6, 12, 18, 24, 30, and 36 months for 1 to 3 years [2]. However, patients with BCG-unresponsive disease - defined as BCG-refractory tumors (T1 high-grade disease after at least 5 out of 6 doses of BCG induction, any high-grade disease during BCG maintenance, carcinoma in situ/Ta high-grade disease after induction, followed by recurrence after reinduction or one maintenance cycle) or early relapse including recurrence with any high-grade disease within 6 months or carcinoma in situ within 12 months after adequate BCG exposure - are unlikely to respond to further BCG alone (BCG reinduction), resulting in the necessity of radical cystectomy (RC) as a next therapeutic step [2].
Various ongoing studies with novel bladder-preserving strategies are therefore currently investigating whether RC can be prevented in BCG-unresponsive patients and whether the BCG-induced antitumor effect can be enhanced in BCG-naïve high-risk NMIBC by combining BCG with immune-enhancing agents, Supplementary Table S1.
A recently published clinical phase 1 trial (ADAPT-BLADDER) was able to show that the combination of the immune checkpoint inhibitor durvalumab and BCG is an effective therapy for BCG-unresponsive NMIBC [3]. In detail, within the durvalumab + BCG cohort, the 3-, and 12-month complete response rate was high with 85% and 73%, respectively [3]. Although this first encouraging preliminary data suggest that combinatory approaches synergistically improve antitumor response of BCG in patients with BCG-unresponsive NMIBC [3], a better understanding of the immunological mechanisms underlying BCG activity and other combination agents is essential for patient selection, biomarker and future drug development.
BCG is a very special vaccine because of its ability to reprogram macrophages metabolically and epigenetically. As a result, repetitive administration of BCG increases macrophage responsiveness, a phenomenon referred to as “trained immunity”. BCG is an effective stimulus for inducing trained immunity. Upon detection of BCG by pattern recognition receptors [4], activation of the Akt/mTOR pathway is crucial to shift cellular metabolism towards glycolysis and glutaminolysis, which - in turn - are required for the induction of trained immunity in human monocytes by BCG. Moreover, epigenetic mechanisms regulate the induction of these pathways at the level of chromatin organization. Specifically, an increase of H3K4me3, a histone mark denoting open chromatin and increased gene transcription, and a decrease of the repressor mark H3K9me3 occurs at the promoters of tumor necrosis factor (TNF)-α and interleukin IL-6 [5].
Clinical and immunological investigations of BCG-induced trained immunity were initially prompted by epidemiological observations, indicating that BCG vaccination can reduce infant mortality from infections other than tuberculosis [6]. BCG-induced protection was particularly related to respiratory tract infections. Further studies demonstrated that BCG-induced immune training can decrease viremia, increase the production of cytokines, and thus expedite the clearance of viruses [7]. The protective effect of BCG-induced trained immunity against respiratory infections including SARS-CoV-2 was also confirmed in bladder cancer patients [8, 9]. These findings clearly indicate that local administration of BCG in the bladder translates into trained immunity at the systemic level.
In addition to the antivirus effect of BCG, there is now first evidence that BCG-induced trained immunity also drives antitumor immune responses in bladder cancer [9-11]. In support of this concept, germline variation in genes that affect trained immunity are linked with recurrence and progression after BCG in NMIBC [9]. Likewise, in a first small study with seven NMIBC patients epigenetic profiling in circulating monocytes has been used to show that BCG response is associated with accumulation of histone trimethylation (H3K4me3) at specific gene loci [11]. Moreover, in a mouse model of NMIBC, systemic immune activation by intravenous administration of BCG as opposed to local administration by bladder instillation also promotes anti-tumor responses [10]. Furthermore, BCG-induced trained immunity facilitates the development of anti-tumor adaptive immunity [10].
Intriguingly, other macrophage functions can also be trained. During infections, macrophages use Fc receptors (FcR) to recognize the Fc region or tail region of antibodies when they are bound to pathogen structures. The subsequent FcR-mediated phagocytosis of immunoglobulin G (IgG)-coated targets serves pathogen clearance in the periphery and may likewise contribute to tumor cell removal [12]. A new study has now demonstrated that prior FcR activation makes macrophages more likely to phagocytose IgG-coated tumor cells upon re-encounter [13]. In other words, macrophages with prior subthreshold Fc receptor activation “eat” more IgG-bound human cancer cells. This work demonstrates that IgG primes macrophages for increased phagocytosis, suggesting that therapeutic antibodies may become more effective after initial priming doses, very similar to BCG priming for enhanced cytokine production, Figure 1.
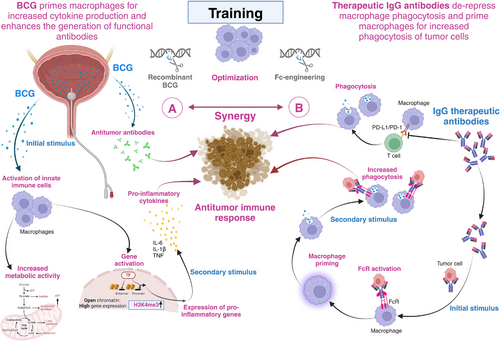
Training macrophage functions synergistically by BCG and immune checkpoint-blocking antibodies to enhance antitumor immune response in bladder cancer. (A) BCG primes macrophages for increased cytokine production and supports the generation of functional antibodies. After an initial stimulus, such as BCG, trained immunity is defined as an enhanced innate immune response to various pathogens. In innate immune cells, especially in macrophages, certain microbial ligands that bind to pattern recognition receptors can cause long-lasting metabolic and epigenetic alterations. When the cell is stimulated again, transcription factors can more easily access the promoter and enhancer regions of pro-inflammatory genes thanks to this reprogramming of the metabolic and epigenetic landscape. This facilitates the expression of pro-inflammatory genes. When these metabolic and epigenetic effects are combined, the response to a secondary stimulation with the same or a different ligand is enhanced, increasing also anti-tumor immune response as BCG primes macrophages for increased cytokine production. In addition to training innate immune responses, BCG vaccination can also enhance the generation of functional antibodies that may, in principle, also be directed against tumor cells. (B) Therapeutic IgG antibodies against PD-1 or PD-L1 de-repress macrophage phagocytosis and prime macrophages for increased FcR-dependent tumor cell phagocytosis. IgG antibodies support restoration of tumor cell phagocytosis by tumor-associated macrophages. In addition, therapeutic IgG antibodies can prime macrophages for enhanced phagocytosis of tumor cells. This raises the possibility that prior activation of FcR in TAMs by therapeutic checkpoint inhibition antibodies facilitates tumor cell killing mediated by anti-tumor antibodies elicited by BCG vaccination, thus generating a synergistic effect between BCG and ICI. In the future, genetic engineering of BCG and Fc-engineering of IgG antibodies might be attractive and may give the script of bladder cancer immunotherapy a new twist. Figure created with Biorender.com. Abbreviations: BCG, Bacillus Calmette-Guérin; FcR, Fc Receptor; IgG, Immunoglobulin G; PD-1, Programmed Cell Death Protein 1; PD-L1, Programmed Cell Death Ligand 1.
Immune checkpoint-blocking antibodies that are currently being tested for combination with BCG are pembrolizumab, sasanlimab, durvalumab and atezolizumab, which activate T cells by blocking the suppressive programmed cell death ligand 1/programmed cell death protein 1 (PD-L1/PD-1) signaling. While T cell suppression by PD-L1/PD-1 signaling is well studied, less is known about the role of this signaling pathway in tumor-associated macrophages (TAMs). A well-established effect of PD-1 activation in TAMs is the suppression of phagocytosis. The phagocytic potency against tumor cells has been shown to be negatively correlated with TAM PD-1 expression, and macrophage phagocytosis was increased when PD-1/PD-L1 was blocked in vivo [14]. These observations indicate that immune checkpoint-blocking antibodies not only serve to facilitate T cell activation but also support the restoration of tumor cell phagocytosis by TAMs, representing yet another mechanism of immune checkpoint inhibitor to enhance antitumor effects [14]. Moreover, once TAM phagocytosis is restored, the IgG training effect can start to work, making tumor cell phagocytosis even more effective [13]. FcR effects are not restricted to phagocytosis but may include cytokine production as well as antibody-dependent and FcR-mediated killing of tumor cells [11]. Additional studies are required to clarify whether these functions can also be trained by prior FcR activation. In addition to training innate immune responses, BCG vaccination can also enhance the generation of functional antibodies [15] that may, in principle, also be directed against tumor cells. This raises the possibility that prior activation of FcR in TAMs by therapeutic checkpoint inhibition antibodies facilitates tumor cell killing mediated by anti-tumor antibodies elicited by BCG vaccination, thus generating a synergistic effect between BCG and ICI.
In any case, it can be summarized that there is a strong incentive to further study the crosstalk between BCG and IgG therapeutic antibodies to enhance trained immunity-mediated antitumor immune response in bladder cancer. While the low-tech vaccine BCG trains macrophages in proinflammatory cytokine production, high-tech immune checkpoint-blocking antibodies restore and train tumor cell phagocytosis by TAM.
Training the synergy between BCG and immune checkpoint-blocking antibodies empowers macrophages to achieve their full potential in the fight against bladder cancer and may be further improved at different levels: on the one hand, BCG can be made more effective for instance through genetic engineering [16], and, on the other hand, Fc-engineering of immune checkpoint-blocking antibodies may help to enhance the training effect of tumor cell phagocytosis by pre-activated macrophages [17]. In continuation of these considerations, physically conjugating immune checkpoint-blocking antibodies to BCG might be attractive and may give the script of bladder cancer immunotherapy a new twist.
AUTHOR CONTRIBUTIONS
Conception and design: Renate Pichler and Martin Thurnher. Acquisition of data: Renate Pichler and Martin Thurnher. Literature search: Renate Pichler and Martin Thurnher. Writing the draft and revision of the manuscript: Renate Pichler and Martin Thurnher. Final approval of the version to be published: Renate Pichler and Martin Thurnher. Both authors have read, reviewed and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
FUNDING INFORMATION
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.
Not applicable.




